| **Institutional Review Board** |
|---|
| University of Nebraska Medical Center |
| 987830 Nebraska Medical Center |
| Omaha, NE 68198-7830 |
| Phone: 402-559-6463 Email: irbora@unmc.edu |
Note: This research will subsequently be divided into therapeutic or non-therapeutic research. Therapeutic research is characterized as research which involves a drug, medical device, technique or other intervention or strategy (including means like diet, cognitive therapy, behavioral therapy, exercise) intended to diagnose, treat or prevent a particular condition or disease.
## Human Biological Material (HBM) application: Research involving the collection of human biological material (HBM) for use for this specific research project, or use of existing HBM (for example, from a biorepository) for a specific research project, or both, with no other type of physical interventions.Note: HBM studies as described above which would additionally include the collection of medical information via surveys or by chart review should also be submitted using this application. If the sole purpose of this project is to obtain IRB approval for establishment of a tissue bank, complete the Tissue Banking Application.
## Tissue Bank application: Collection of human biological material (HBM) with no other type of physical or laboratory testing for the sole purpose of using the samples for FUTURE research.Note: If the intent of the protocol is to collect HBM for use for this specific research with storage of left-over material after completion of this specific research, use the Human Biological Material (HBM) application.
## Medical Records application: Research involving the collection of information from medical records whether or not the information already exists or will be recorded in the future.Note: Medical records research should not be submitted using the Exempt Application.
## Data Registry application: The collection of information/data (typically from medical records) for the sole purpose of using the data for FUTURE research. ## Exempt Research application: ***This application is only use for research which falls into specifically defined categories under the federal regulations (as outlined below). DO NOT use this application for medical record research.*** 1. Research conducted in established or commonly accepted educational settings that specifically involves normal educational practices. 2. Research that only involves educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior (including visual or auditory recording). 3. Research involving benign behavioral interventions in conjunction with the collection of information from an adult subject through verbal or written responses. 4. Research uses of identifiable private information (either publicly available or subsequently de-identified) that has been collected for some other activity. 5. Research and demonstration projects that are conducted or supported by a Federal department and are designed to study, evaluate, improve, or otherwise examine public benefit or service programs. 6. Taste and food quality evaluation and consumer acceptance studies.Note: The Office of Regulatory Affairs (ORA) and the IRB have sole authority to determine whether a research project satisfies requirements for exemption. Contact the IRB Office if you have any questions - \[irbora@unmc.edu\](mailto:irbora@unmc.edu).
## Humanitarian Use Device (HUD) application: Use of a Humanitarian Use Device (HUD) which is a device that is intended to benefit patients in the treatment and diagnosis of diseases or conditions that affect fewer than 8,000 individuals in the United States per year.Note: This application should not be used if the HUD is the subject of a clinical investigation in which safety and effectiveness data is being collected to support a pre-marketing approval (PMA) application. The IRB Application for Biomedical Research should be used instead.
## Central IRB (cIRB)/Single IRB (sIRB) application: Multisite research where UNMC will be relying on another IRB for review. This includes commercial IRBs like Advarra and CG-WIRB, Consortium or other Network IRBs, NIH or federal agency IRBs like the NCI CIRB, or other academic institutions. Click [here](https://guides.unmc.edu/books/institutional-review-board-irb-guidebook/chapter/single-central-irb) for more information regarding cIRB and sIRB protocols or email| ***Submission Deadline*** | ***Date of Adult Meeting*** |
|---|---|
| 2/7/2025 | 2/20/2025 |
| 2/21/2025 | 3/6/2025 |
| 3/7/2025 | 3/20/2025 |
| 3/21/2025 | 4/3/2025 |
| 4/4/2025 | 4/17/2025 |
| 4/18/2025 | 5/1/2025 |
| 5/2/2025 | 5/15/2025 |
| 5/23/2025 | 6/5/2025 |
| 6/6/2025 | 6/19/2025 |
| 7/3/2025 | 7/17/2025 |
| 7/25/2025 | 8/7/2025 |
| 8/8/2025 | 8/21/2025 |
| 8/22/2025 | 9/4/2025 |
| 9/5/2025 | 9/18/2025 |
| 9/19/2025 | 10/2/2025 |
| 10/3/2025 | 10/16/2025 |
| 10/24/2025 | 11/6/2025 |
| 11/7/2025 | 11/20/2025 |
| 11/21/2025 | 12/4/2025 |
| 12/5/2025 | 12/18/2025 |
| 12/31/2025 | 1/15/2026 |
| ***Submission Deadline*** | ***Date of PEDs Meeting*** |
|---|---|
| 2/13/2025 | 2/25/2025 |
| 3/13/2025 | 3/25/2025 |
| 4/10/2025 | 4/22/2025 |
| 5/15/2025 | 5/27/2025 |
| 6/12/2025 | 6/24/2025 |
| 7/10/2025 | 7/22/2025 |
| 8/14/2025 | 8/26/2025 |
| 9/11/2025 | 9/23/2025 |
| 10/16/2025 | 10/28/2025 |
| 11/13/2025 | 11/25/2025 |
| 12/4/2025 | 12/16/2025 |
| 1/15/2026 | 1/27/2026 |
| ***Submission Deadline*** | ***Date of sIRB Meeting*** |
|---|---|
| 2/6/2025 | 2/14/2025 |
| 3/6/2025 | 3/14/2025 |
| 4/3/2025 | 4/11/2025 |
| 5/1/2025 | 5/9/2025 |
| 6/5/2025 | 6/13/2025 |
| 7/3/2025 | 7/11/2025 |
| 7/31/2025 | 8/8/2025 |
| 9/4/2025 | 9/12/2025 |
| 10/2/2025 | 10/10/2025 |
| 11/6/2025 | 11/14/2025 |
| 12/4/2025 | 12/12/2025 |
| 12/31/2025 | 1/9/2026 |
Note: For Student Principal Investigators, if your research study will be registered on ClinicalTrials.gov, please list your Faculty Advisor as the Responsible Party and yourself as Record Owner.
*** ##### CITI Training Course for ClinicalTrials.gov A new CITI training course is available to UNMC and UNO learners that provides video instructions for registering, uploading documents, and submitting results in ClinicalTrials.gov. Currently optional, the course is highly recommended as a guide to investigators new to ClinicalTrials.gov requirements and to experienced investigators needing a refresher. * Login into your UNMC or UNO [CITI account](https://www.citiprogram.org/index.cfm?pageID=14&_ga=2.146561085.887993944.1722258539-100311130.1720707725) * Scroll down to 'Add a Course' * Click the box for Protocol Registration and Results Summary Disclosure in ClinicalTrials.gov * Click 'Next'. When you have successfully completed the course, please email a copy of the Completion Certificate to [oract.gov@unmc.edu](mailto:oract.gov@unmc.edu). *** ##### ClinicalTrials.gov Icons in RSS IRB protocols that are investigator-initiated and registered on ClinicalTrials.gov with an NCT# will now be denoted by an icon in RSS. A green icon means no problems are currently identified by ClinicalTrials.gov on the record associated with the study.  A red icon means ClinicalTrials.gov has identified problems on the associated record.  If your study has a red icon, please login at [https://register.clinicaltrials.gov](https://register.clinicaltrials.gov) to correct the problem(s). Icons are updated daily, Monday-Friday, so once problems are resolved, the icon will be green after the next daily update. If you have difficulty correcting a problem in [https://register.clinicaltrials.gov](https://register.clinicaltrials.gov), please contact [oract.gov@unmc.edu](mailto:oract.gov@unmc.edu) for assistance. Outstanding problems with the ClinicalTrials.gov record may delay the review and approval of IRB submissions. Please ensure all problem records are addressed as soon as possible ([UNMC HRPP Policy 1.29](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/129-clinicaltrialsgov-reporting)). *** ##### Process for updating a record Whenever a ClinicalTrials.gov record is updated, the process must be completed by approving and releasing the update. Steps for completing an update of any type are displayed in the “Record Status” at the top of the record.  The “Next Step” box is displayed immediately below which describes the next action needed. Any problems with the update are listed in the “Next Step” box and may include: * Correct Error(s) * Enter Results * Finish Protocol/Documents/Results section * Address Review Comments Once the update problems are resolved, the next step is to click the “Entry Complete” button:  The following step is to review the update, then click the “Approve” button:  The last step is to click the “Release” button if you are the Responsible Party for the record:  If you are the Record Owner, this “Next Step” box will be displayed, and the Responsible Party will need to login and release the update.  When any problems with the update are resolved and the update has passed PRS review, the update is released to the public ClinicalTrials.gov site.  All the steps on the “Record Status” will be highlighted in blue. *** ##### Posting Consent Forms For any clinical trial conducted or supported by a federal agency or department or agency, Federal Regulations require the awardee of a grant to post one IRB approved informed consent form used to enroll subjects on a publicly available Federal Web site. “Clinical trial” means a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of the interventions on biomedical or behavioral health-related outcomes. The PI must post: * Only if the organization (UNMC, Nebraska Medicine, UNO or CHMC) is the lead site or grantee organization. * Only clinical trials (defined above). As a general rule, if you reported to clinicaltrials.gov then you will also have to post the consent form. * Only if conducted or supported by a Federal department or agency. The PI only needs to post **ONE** consent form used to enroll subjects anytime during the course of the study. The consent form must be posted no later than 30 days after the last subject is enrolled. If your study is utilizes the Clinical Trials Monitoring System (CTMS) you will receive notification when your last subject is enrolled. The notification includes instructions about the requirement, and how to post to [clinicaltrials.gov](https://clinicaltrials.gov/). If your study does not utilize CTMS it is your responsibility to track subject enrollment, and post no later than 30 days after the last subject is enrolled. Specific instructions on how to register with ClinicalTrials.gov and upload documents can be found [here](https://clinicaltrials.gov/ct2/manage-recs). # Emergency Treatment The contact list for Emergency Treatment authorization can be found here in [RSS](https://net.unmc.edu/rss/rss_irb_chair_schedule.php). Under certain circumstances, a physician may treat a patient with an investigational (non-FDA approved) drug, biologic or device, or treat a patient utilizing a non-IRB approved protocol; Pursuant to FDA regulations, the patient must be suffering from a serious, life-threatening or debilitating illness for which there is no satisfactory treatment alternative(s) and there must not be sufficient time to obtain full IRB review and approval. Emergency treatment as defined here is not research. The FDA regulations do not provide for expedited IRB approval in emergency situations. UNMC/Nebraska Medicine policy requires the IRB be notified prior to such use, by contacting the IRB office. This notification is not IRB approval. The IRB will only state it is aware of the proposed use and considers the use to meet the requirements of 21 CFR 56.102(d), 21 CFR 56.104(c), and the criteria in [HRPP 6.4 Emergency Use of a Test Article](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/64-emergency-use-of-a-test-article), section 5.0 of the full policy. The investigator is still required to obtain informed consent of the patient or the patient’s legally authorized representative. The consent form must contain appropriate elements structured to reflect that consent is for treatment purposes as opposed to research. View a sample [Consent Form for Emergency Treatment](https://guides.unmc.edu/attachments/22) A useful guide can be found here: [Emergency Use vs Expanded Access](https://guides.unmc.edu/attachments/23) The UNMC Emergency Use of a Test Article Report form can be found in RSS and a signed copy of the consent form must be submitted to the IRB within 5 business days following the treatment. # Exempt Studies It is understood this project will be conducted in full accordance with all applicable HRPP Policies. It is also understood that the ORA will be immediately notified of any proposed changes for your research project that: 1. Affect the risk-benefit relationship of the research 2. Pose new risks which are greater than minimal 3. Constitute a new risk to privacy or confidentiality 4. Involve sensitive topics (including but not limited to personal aspects of the subjects behavior, life experiences or attitudes) 5. Involve deception 6. Target a vulnerable population 7. Include prisoners or children 8. Otherwise suggest loss of the exempt status of the research. No changes to exempt studies are required to be submitted for review, which is outlined in your approval letter. If you feel a change is warranted, please contact our office utilizing the message portal of the application in question. You are encouraged to contact the ORA to discuss whether changes to exempt research requires review by the IRB or please view [HRPP policy 2.6](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/26-exempt-research) (Exempt Research). # Incidents (Non-compliance/Problems) Per [HRPP policy 8.4](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/84-review-of-noncompliance-involving-the-pi-or-study-personnel) non-compliance (NC) involving the PI and/or study personnel must be promptly reported to the IRB. This non-compliance could involve failure to comply with the Federal Regulations related to the protection of human subjects of research, HRPP policies, or the requirements/determinations of the IRB. Research related problems are incidents that do not involve NC on the part of the PI and/or study personnel. These occur during the course of the study and may include things such as: subject incorrectly adhering to instructions (taking a wrong dosage of study drug), research records being stolen/lost, etc. Research related problems must also be promptly reported to the IRB. NC and problems are reported to the IRB by submission of an Incident Report Form. The Incident Report form is located in the Forms section of [RSS](https://net.unmc.edu/rss/). The PI is responsible for ensuring that the required reports are submitted promptly following discovery of the incident in accordance with [HRPP policy 8.4](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/84-review-of-noncompliance-involving-the-pi-or-study-personnel). *If you have any questions regarding this submission process, please contact the IRB Office at [irbora@unmc.edu](mailto:irbora@unmc.edu)* # Protocol Deviations A Single Subject Protocol deviation is a change in an IRB-approved protocol which is permitted for an individual subject when it is in the best interest of that subject and/or is necessary for research purposes (e.g., data completion). Protocol deviations are classified as either “minor” or “more than minor.” Once the form is received, the deviation will be reviewed and processed by the IRB. Deviations may be approved in one of two ways: 1. Deviations that are ***minor*** are eligible for expedited review under the provisions of HHS regulations at 45 CFR 46.110(b)(2) and FDA regulations at 21 CFR 56.110(b)(2), as applicable. 2. Deviations that are ***more than minor*** do not qualify for expedited review and therefore must be reviewed by the full IRB. Once the deviation is approved, an approval letter for the Single Subject Protocol Deviation will be attached to the electronic IRB application. The PI and Lead Coordinator will be notified via email when this occurs. To obtain Single Subject Protocol Deviation approval, a Single Subject Protocol Deviation Request must be submitted PRIOR to the implementation of the deviation. The instructions for submission are below: **RSS Studies & Existing Paper Format Studies** The Single Subject Deviation form can be created by pulling up the protocol in RSS. The 'Forms' section will be found in the left-side menu. When clicking on 'Forms' a list will appear allowing you to choose the 'single subject protocol deviation request'. Please follow the instructions provided to complete the form and once competed the PI will electronically sign and click 'SUBMIT'. *If you have any questions regarding this submission process, please contact the IRB Office at [irbora@unmc.edu](mailto:irbora@unmc.edu).* # Submission Process ## Full Board Review (initial submission) Investigators will be notified of the assigned IRB# by email. Applications for Full Board Review will be reviewed at the next possible IRB meeting. Submission Deadlines can be found [here](https://guides.unmc.edu/books/institutional-review-board-%28irb%29-guidebook/page/submission-deadlines). These documents must also be attached in the "ADD DOCUMENTS" section of the online application at the time of electronic submission: New Submissions (Initial) 1. Subject Recruitment Material (as applicable) 2. External study site approval letters (as applicable) 3. Full protocol (as applicable) 4. Investigator's Brochure (as applicable) 5. Grant application (as applicable) 6. Clinical Trial Master Matrix (as applicable) 7. Other relevant material (e.g. surveys) (as applicable) Tabled — Re-Submissions 1. Investigator's response letter 2. Other revised materials (as applicable) ## Expedited Review (initial submission) Certain studies involving no more than minimal risk may qualify for expedited review status under 45 CFR 46.110 or 21 CFR 56.110. View a [list of categories](http://www.hhs.gov/ohrp/policy/expedited98.html) which may qualify for expedited review. Expedited review of **new** protocols are handled through the electronic submission only. New submissions eligible for expedited review will be reviewed by the IRB Analyst with appropriate confirmation by the Executive Chair/designee and the investigator will be informed of the IRB’s decision by email. ## Exempt Protocols (initial submission) Research activities in which the only involvement of human subjects will be in one or more of the categories specified by 45 CFR 46.101(b) are exempt from the requirements of 45 CFR 46. The exempt categories do not, however, apply to research involving deception of subjects, sensitive behavioral research, or to research involving pregnant women, prisoners, individuals who are decisionally impaired and other subject populations determined to be vulnerable. Reviews of new Exempt protocols are handled through the electronic submission only. New Exempt submissions will be reviewed by a member of the Office of Regulatory Affairs (ORA) staff and the investigator will be informed of the ORA's decision by email. ## Request for Change Any proposed change in a research activity must be reviewed and approved by the IRB prior to implementation **except** when: 1) a change is necessary to eliminate an apparent immediate hazard to the subject(s), or 2) a subject needs to be advised immediately of significant new information. Administrative changes do not require IRB review and can, accordingly, be approved by ORA. **For studies submitted in electronic system**, follow these steps: * Reset the application to Edit * Make changes to the IRB application and click SAVE * Revise consent documents (if applicable) and click COMPLETE * Upload any applicable documents using the Add Document function * Click CHANGE REQUEST on the left-side menu and complete * Sign the application (PI) * Click SUBMIT **For Studies NOT Submitted in Electronic System**, follow these steps: * Complete, sign (PI), and upload the request for change form found [here](https://guides.unmc.edu/books/institutional-review-board-%28irb%29-guidebook/page/existing-paper-protocols). * Revise the current approved application using track changes * Sign Section I of the application (PI) * Revise current approved Consent document as applicable using tracked changes * Complete and sign the appropriate Request for Change * Upload all documents associated with this change (e.g., application, consents, Request for Change, etc) using the Add Document function If you have any questions regarding this submission process, please contact the [IRB Office](mailto:irbora@unmc.edu). ## Continuing Review Overview Federal regulations require certain types of research undergo continuing review at least annually. Review and approval of continuing review must occur before the expiration date listed on the initial approval letter and, subsequent continuing review approval letters as the “valid until” date. Courtesy email reminder notifications are sent approximately two months before expiration and again two weeks later. Federal regulations prohibit the IRB from granting extensions or temporary approval beyond the expiration date. Should expiration of IRB approval occur, all study related activities must cease as of the date of expiration. A “Request to Continue Treatment for Enrolled Subjects on Approval Expired Studies” form must be submitted and approved to allow currently enrolled subjects to continue to receive study treatment. This form is available through the “Forms” link on study pages in RSS. **CR Electronic Submission** * Complete, sign (PI), and submit the continuing review form within RSS (located on the left side menu under “Forms”) * Upload the following documents, as applicable, using the “Add Document” function: * Last signed consent form (only if a subject was consented or reconsented since the last continuing review). **These are no longer to be redacted** (i.e. subject identifiers do not need to be blacked out). * Scientific Review Committee (SRC) CR approval letters * DSMB or other safety review reports * Progress reports * Publications **CR Existing Paper Protocol Submissions** * Complete, sign (PI), and upload the continuing review form found [here](https://guides.unmc.edu/books/institutional-review-board-%28irb%29-guidebook/page/existing-paper-protocols). * Upload the following documents, as applicable, using the “Add Document” function: * Consent documents (Word format and clean to be date stamped) * Last signed consent form (only if a subject was consented or reconsented since the last continuing review). **These are no longer to be redacted** (i.e. subject identifiers do not need to be blacked out). * Scientific Review Committee (SRC) CR approval letters * DSMB or other safety review reports * Progress reports * Publications **Submission Deadlines** Studies requiring Full Board (convened IRB meeting) review, continuing review applications should be submitted 4-6 weeks before expiration. Studies requiring expedited review, continuing review applications should be submitted four weeks prior to expiration, to allow time to address any required modifications. Studies that do not require continuing review, are required to submit an annual update, which includes a report of subject demographics. Emails requesting the annual update are sent out the month the study will expire. If you have any questions regarding this submission process, please contact the IRB Office at [irbora@unmc.edu](mailto:irbora@unmc.edu). # Education & Resources The goal of the IRB Education Program is to facilitate research involving human subjects from initial submission to study completion through didactic and practical education. Whether it is for investigators, research study personnel, students or other institutional representatives, information is explained in a manner that fits the audience. By using a variety of delivery methods, such as lectures, webinars, live-streams, bulletins, one-on-one meetings and department in-service, from new student to seasoned investigator, our objective is to offer education of:- The history and regulation of research ethics- The local submission requirements and process- The common pitfalls to improve the efficiency of the submission process. The IRB is here to help!All educational options will be tailored to meet the specific needs of the target audience, whether it be one-on-one about a specific research protocol or a lecture to a class regarding a general overview of research ethics and the IRB process.If you would like to schedule a one-on-one meeting, department in-service, class lecture, Q&A session or any other type of IRB education, please contact IRB staff for assistance at irbora@unmc.edu # HRPP Investigator Guidance Series This page serves as a hub for all of the Investigator Guidance Series documents. Each document is an abbreviated version of one of our [HRPP Policies and Procedures](https://guides.unmc.edu/books/hrpp-policies-and-procedures) intended for investigators, coordinators, and other study team members. This page is a good starting point for any study team member with a question about a policy on a specific topic. A link to the full policy/procedure is included in each document. | Document | Updated | |----------|---------| |[Advertisements (3.5)](https://guides.unmc.edu/attachments/25)|12/13/2023| |[Authority of the IRB](https://guides.unmc.edu/attachments/26)|12/13/2023| |[Change Requests](https://guides.unmc.edu/attachments/24)|12/13/2023| |[cIRB](https://guides.unmc.edu/attachments/28)|12/13/2023| |[Closure of Research](https://guides.unmc.edu/attachments/29)|12/13/2023| |[Compensation](https://guides.unmc.edu/attachments/202)|12/09/2024| |[Confidentiality](https://guides.unmc.edu/attachments/31)|12/13/2023| |[Continuing Review](https://guides.unmc.edu/attachments/32)|02/05/2024| |[Contraception Requirements](https://guides.unmc.edu/attachments/33)|12/13/2023| |[Data and Safety Monitoring](https://guides.unmc.edu/attachments/34)|12/13/2023| |[Data Registries](https://guides.unmc.edu/attachments/35)|12/15/2023| |[Emergency Research - Waiving Consent](https://guides.unmc.edu/attachments/36)|12/13/2023| |[Emergency Use of a Test Article](https://guides.unmc.edu/attachments/37)|01/25/2024| |[Employees as Subjects](https://guides.unmc.edu/attachments/38)|12/13/2023| |[Ethical Access](https://guides.unmc.edu/attachments/203)|12/09/2024| |[Exempt Research](https://guides.unmc.edu/attachments/39)|01/25/2024| |[Expanded Access to Investigational Drugs and Devices](https://guides.unmc.edu/attachments/40)|12/14/2023| |[Expedited Review](https://guides.unmc.edu/attachments/41)|12/13/2023| |[Financial COIs](https://guides.unmc.edu/attachments/42)|01/25/2024| |[Full Board Review](https://guides.unmc.edu/attachments/43)|12/13/2023| |[Humanitarian Use Device (HUD)](https://guides.unmc.edu/attachments/44)|12/13/2023| |[Incidental Findings](https://guides.unmc.edu/attachments/45)|12/13/2023| |[Increased monitoring, interim Continuing Review, and verification](https://guides.unmc.edu/attachments/46)|12/15/2023| |[Informed Consent](https://guides.unmc.edu/attachments/47)|12/13/2024| |[International Research](https://guides.unmc.edu/attachments/48)|12/13/2023| |[Investigational and Marketed Devices](https://guides.unmc.edu/attachments/49)|01/26/2024| |[Investigational and Marketed Drugs](https://guides.unmc.edu/attachments/50)|12/13/2023| |[IRB Approval Criteria](https://guides.unmc.edu/attachments/51)|12/15/2023| |[Obtaining Informed Consent for Non-English Speaking Persons](https://guides.unmc.edu/attachments/52)|12/13/2023| |[Obtaining Informed Consent for Persons with Additional Needs](https://guides.unmc.edu/attachments/53)|12/13/2023| |[PI Qualifications and Responsibilities (job description)](https://guides.unmc.edu/attachments/54)|12/13/2023| |[Placebos](https://guides.unmc.edu/attachments/55)|12/13/2023| |[Post-Approval Monitoring of Research](https://guides.unmc.edu/attachments/204)|12/09/2024| |[Pregnancy Testing](https://guides.unmc.edu/attachments/56)|12/13/2023| |[Privacy](https://guides.unmc.edu/attachments/57)|01/26/2024| |[Recruitment](https://guides.unmc.edu/attachments/58)|12/13/2023| |[Reimbursement](https://guides.unmc.edu/attachments/59)|12/13/2023| |[Research Involving Children](https://guides.unmc.edu/attachments/60)|12/13/2023| |[Research Involving Decisionally Impaired Persons](https://guides.unmc.edu/attachments/61)|12/13/2023| |[Research Involving Neonates](https://guides.unmc.edu/attachments/62)|12/13/2023| |[Research Involving Pregnant Women and Fetuses](https://guides.unmc.edu/attachments/63)|12/13/2023| |[Research Involving Prisoners](https://guides.unmc.edu/attachments/64)|01/25/2024| |[Research Personnel Qualifications & Responsibilities (study team job descriptions)](https://guides.unmc.edu/attachments/65)|12/13/2023| |[Short Form Consent](https://guides.unmc.edu/attachments/66)|12/13/2023| |[sIRB](https://guides.unmc.edu/attachments/67)|12/13/2023| |[Students as Subjects](https://guides.unmc.edu/attachments/68)|12/13/2023| |[Study Hold](https://guides.unmc.edu/attachments/69)|12/13/2023| |[Suspension](https://guides.unmc.edu/attachments/70)|12/13/2023| |[Termination](https://guides.unmc.edu/attachments/71)|12/13/2023| |[Using PHI in Research](https://guides.unmc.edu/attachments/72)|12/13/2023| |[Vulnerable Populations - Additional Protections](https://guides.unmc.edu/attachments/73)|12/13/2023| |[Waiving Consent Process](https://guides.unmc.edu/attachments/74)|12/13/2023| |[Waiving Signed Consent](https://guides.unmc.edu/attachments/75)|12/13/2023| |[Wash Out](https://guides.unmc.edu/attachments/76)|12/13/2023| |[What requires IRB review and approval?](https://guides.unmc.edu/attachments/27)|01/10/2024| # Investigator Resources ## Regulations: * [Common Rule (45 CFR 46)](https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html) * [eCFR: 21 CFR Part 50-Protection of Human Subjects](https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-50) * [eCFR: 21 CFR Part 56-Institutional Review Boards](https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-56) ## National Institutes of Health: * [NIH Home Page](https://www.nih.gov/) * [Office of Recombinant DNA Activities (ORDA)](https://osp.od.nih.gov/wp-content/uploads/NIH_Guidelines.pdf) * [Office of Grants and Contracts](https://grants.nih.gov/grants/oer.htm) * [National Human Genome Research Institute](https://www.genome.gov/) * [Ethical, Legal and Social Implications (ELSI)](https://www.genome.gov/10001740/ethical-legal-and-social-issues-in-genomic-medicine/) ## UNMC Links: * [General Counsel's Memo on Mandatory Reporting of Child Abuse and Related Statute of Limitations](https://www.unmc.edu/irb/resources/_files/child-abuse-reporting-memo-unmc-04032013.pdf) * [Institutional Biosafety Committee (IBC)](https://www.unmc.edu/ibc/) * [Animal Care and Use Program (IACUC)](https://info.unmc.edu/comparativemed/) * [Sponsored Programs Administration (SPA)](http://www.unmc.edu/spa/) ## Food and Drug Administration: * [FDA Web Site](http://www.fda.gov/) * [FDA - Center for Drug Evaluation and Research (CDER)](http://www.fda.gov/cder) * [FDA - Center for Devices and Radiological Health](https://www.fda.gov/about-fda/fda-organization/center-devices-and-radiological-health) ## International Standards: * [International Compilation of Human Research Standards](http://www.hhs.gov/ohrp/international/intlcompilation/intlcompilation.html) ## Other Federal Agencies: * [National Archive and Records Administration](https://www.archives.gov/) * [Federal Register Online](https://www.federalregister.gov/) * [Office for Civil Rights](https://www.hhs.gov/ocr/index.html) * [Medical Privacy - National Standards to Protect the Privacy of Personal Health Information](http://www.hhs.gov/hipaa) * [Department of Health and Human Services](https://www.hhs.gov/) * [Child Abuse Reporting Memo](https://guides.unmc.edu/attachments/77) ## Organizations and Other Items of Interest: * [Public Responsibility in Medicine & Research (PRIM&R)](http://www.primr.org/) * [National Bioethics Advisory Commission (NBAC)](https://bioethicsarchive.georgetown.edu/nbac/pubs.html) * [American Society for Bioethics and Humanities](http://www.asbh.org/) ## IRB History and Principles: * [“What Makes Clinical Research Ethical?”](https://www.unmc.edu/irb/resources/_files/EmanuelClinicalResearchEthical.pdf) Emanuel, et al; JAMA 283(20):2701, 2000 * [THE BELMONT REPORT: Ethical Principles and Guidelines for the Protection of Human Subjects of Research(UNMC)](https://www.unmc.edu/irb/resources/_files/the-belmont-report-508c_FINAL.pdf) * [The Belmont Report(HHS)](http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.html) # IRB Conference Content # 2024 * [2024 IRB Conference Introduction (Bruce Gordon)](https://echo360.org/media/603718e5-c010-4836-ae65-046c009f0e14/public) * [2024 IRB Conference Conclusion (Bruce Gordon)](https://echo360.org/media/70b907d9-ed82-4681-b421-e55c650bfc6e/public) * [Big Data and Ethics (Scott Campbell)](https://echo360.org/media/ded87d15-1eb2-4405-bec6-154b616c13d5/public) * [Considerations for Patient Safety in Studies of Psychedelics and other Non-Ordinary States of Consciousness. (Lou Lukas)](https://echo360.org/media/5faed299-8d3f-47fc-ac08-86a9d33ce261/public) * [De-Centralized Trials (Megan Singleton)](https://echo360.org/media/0367986b-caf4-4b6f-8716-b059ebf4fb5a/public) * [Let's Review Some Research (Nancy Olson/Nichelle Cobb)](https://echo360.org/media/0d6603d9-e01e-41c9-87b5-093316b43b1d/public) * [Research on the Edge (Bruce Gordon)](https://echo360.org/media/d3f3c6e5-6fae-4476-b36c-86e6c9309c8f/public) # 2023 * [2023 IRB Conference Introduction (Bruce Gordon/Russell McCulloh)](https://echo360.org/media/9a48e0a2-aca1-4487-aff5-357bec3168c2/public) * [Participant Compensation (Joe Brown/Dustin Krutsinger)](https://echo360.org/media/db57777f-2036-4b8a-8749-559c4356fa63/public) * [Personal Narrative and Research Ethics (Gigi McMillan)](https://echo360.org/media/dd6982b9-4ba5-4b06-b673-eb3eb2822942/public) * [Re-examining the IRB's Role in Protecting Research Subjects (David Strauss)](https://echo360.org/media/5cad5336-6560-4347-a018-85fd0d01041a/public) * [Uncovering Bias in Artificial Intelligence (John Windle)](https://echo360.org/media/9a93c457-daf3-40f2-95f3-b22c03ae9c45/public) * [Understanding CBPR (Keyonna King/Russell McCulloh)](https://echo360.org/media/fde28622-0e29-4003-a3e2-a6942528be23/public) # 2022 * [2022 IRB Conference Introduction (Bruce Gordon)](https://echo360.org/media/ae72d7a0-ca24-4bbc-bc3f-27eec7c5fe97/public) * [Genetics, Research, and Diversity (Omar Rehman/Kristi DeHaai)](https://echo360.org/media/766c70f2-9980-4be3-9f46-f8230abfea9d/public) * [Informed Consent & Teach Back Workshop (Elizabeth Bankert)](https://echo360.org/media/acda203a-b5a6-4feb-86c5-ad9511a6b100/public) * [Intro to the IRB (Bruce Gordon)](https://echo360.org/media/fa86c3ed-f786-4d3f-9a17-46a9001e34df/public) * [Readability is Fundamental (Nichelle Cobb)](https://echo360.org/media/da6f7dc6-20e8-46a2-96aa-650edbd50c8c/public) * [Review of Research Involving Products with EUA (Kindra Cooper)](https://echo360.org/media/17c46f37-02f2-47f6-a9ce-a43163ce843b/public) * [Risk of Harm to Non-Subjects (David Borasky)](https://echo360.org/media/5df27933-a35f-4390-9770-0ca507c87b40/public) # Mental health considerations ## MENTAL HEALTH CONSIDERATIONS FOR RESEARCHERS `DECEMBER 2023` ### EXPLANATION OF RISKS: * Be clear in application and ICD about risks associated with mental health assessments (cognitive status assessments, IQ screens, mental health assessments, exploitation/abuse/violence assessments, and drug testing). * Describe how and by who mental health assessments and outcomes are reviewed and reported. * Remember to report psychiatric adverse events, including serious adverse events, appropriately. ### SELF-REPORT MEASURES: * Protocols using **subject self-reports** that ask about depression, worthlessness/guilt, and quality of life, should include a process of review by personnel with plan to notify investigator of pertinent positives. * Protocols using **subject self-report** reports with items specifically addressing **self-harm or suicidal ideation, or related items indicating a subject may be at risk**, should have a mechanism for responses to be reviewed in **REAL TIME** so action can be taken as appropriate. * Protocols using remote self-reports (Ipad, EMA device, web-based, etc.) should include a mechanism for notification of the investigator or designated member of the study team when threshold responses are received so that **REAL TIME** management can occur. ### INVESTIGATOR-ADMINISTERED MEASURES: * Investigator-administered measures of psychiatric symptoms should be completed by those with appropriate training. * If the study team does not have the specific expertise, consider consultation with psychiatry or psychology colleagues. ### PHQ-9: * PHQ-9: Suggestion to align with Suicide Risk BPA’s used by NM PCMH clinics rooming staff starting 8/8/2022: * if >14 and + response to question 9 = refer for emergency eval * if >14 and – response to question 9 = refer for mental health consult * if <14 and + response to question 9 = further assessment needed; refer as appropriate * if <14 and – response to question 9 = no further specific intervention ### The Columbia Suicide Severity Rating Scale-Revised (CSSRS-R): * Baseline (“lifetime”) and “since last visit” versions available on-line. * Validated and available in Spanish. * Use of this scale should include training for non-mental health providers as it explores suicidality in a very thorough manner: * To complete the C-SSRS Training for Clinical Practice, visit [http://c-ssrs.trainingcampus.net/](http://c-ssrs.trainingcampus.net/) * General information, go to [http://cssrs.columbia.edu/](http://cssrs.columbia.edu/) ### RESOURCES: * CURRENT – include **988** for the suicide hotline, don’t give numbers to agencies now closed (**911 is still ok to use**). * ACCURATE—know the policy for referral to the Department of Psychiatry, procedures for accessing ER, the Psychiatric Emergency Service (PES). Consider age- and/or diagnosis-appropriate services (e.g. Nebraska Family Help Line [1-888-866-8660]; Professional Partners-Region specific). * LOCAL—while resources are limited in some areas of the state, please make sure you list the ones close to the subject’s home. ### UNMC/NE MEDICINE PSYCHIATRY SERVICES: * Psychiatry (ADULT) accepts referrals from PCP’s within the system. * C/A psychiatry not limited to UNMC/NE Med providers. * Behavioral Health Connections team (402-552-6007) facilitates referrals to community agencies. * When referring to the “PES” (Psychiatric Emergency Service), understand that patients still must go through the regular NE Med ER or Bellevue Medical Center ER first. ### PSYCHIATRY SERVICES FOR CHILDREN * Immanuel (CHI) ER is primary location for inpatient triage for children/teens; other ER’s may transfer there if hospitalization is needed. * Bryan LGH (Lincoln) has inpatient care for children/teens as well as emergency shelter placement. * Boys Town (Grand Island) has emergency shelter placement. * Mercy (Council Bluffs) will accept NE youth (even Medicaid if no NE beds available). * Boys Town has an inpatient unit—triage through Methodist ER’s. ### PSYCHIATRY SERVICES FOR STUDENTS: * **For UNMC students**: call UNO Health Center, 402-554-2374 (select option 2 to leave message for the nurse for scheduling). * For **UNO students**: Call CAPS 402-559-7276 (initial appointments are covered by student fees). * **Gender and Sexuality Resource Center (GSRC)**: Confidential and free, Student Life Center 2031. Call 402-559-7276. ### KEARNEY COMMUNITY RESOURCES * S.A.F.E. Center: 24/7 hotline 1-877-237-2513 ### LINCOLN COMMUNITY RESOURCES * Voices of Hope: Crisis hotline 402-475-7273 (non-emergencies, 402-476-2110) ### NORFOLK COMMUNITY RESOURCES * Bright Horizons: call 877-379-3798 or text 402-370-8817 ### SCOTTSBLUFF COMMUNITY RESOURCES * Doves Program: call 308-436-4357 or 866-953-6837; text 515-599-6620 ### NATIONAL RESOURCES * National Domestic Violence Hotline: 1-800-799-7233, TTY 1-800-787-3224 * National Suicide Prevention Lifeline: Text or Call 988 * Trans Lifeline: 1-877-565-8860 # Miscellaneous Resources Below investigators will find a variety of resources that may assist with research goals: * [Allowable Costs Related to Participant Inclusion Activities](https://guides.unmc.edu/attachments/195) * [Emergency Preparedness / Continuity of Operations Plan (EP/COOP)](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/emergency-preparedness-continuity-of-operations-plan-%28epcoop%29) # Training The topics found below will provide various learning resources for common IRB items.If there is a topic that you would like to learn more about but do not see it listed below, please contact us at 402-559-6463 or at irbora@unmc.edu. # CITI Training [](https://guides.unmc.edu/uploads/images/gallery/2024-07/U5euQJAvlnAhMbkR-citi_logo.png) ## What is CITI? [Collaborative Institutional Training Initiative (CITI)](https://www.citiprogram.org/index.cfm?pageID=14&_ga=2.112680004.1752575867.1721827048-100311130.1720707725) certification is an institutional requirement for all personnel engaging in Human Subject Research (HSR). It includes three primary modules that are required by the institution to participate in conducting various types of research: Group 1: Biomedical Research, Group 2: Good Clinical Practice (GCP), and Group 3: Social & Behavioral Research. Faculty, employees, students and other institutional representatives at UNMC, Nebraska Medicine, CHMC, and UNO are required to complete the Human Subjects Research (HSR) course via CITI if they will be working on a research project that involves human subjects. It takes approximately 1-2 hours to complete a Basic course. The training does not have to be completed in one sitting, but can be spread out over time if needed. ## Instructions A PDF with instructions for how to navigate CITI can be found [here](https://guides.unmc.edu/attachments/13). **Basic or Refresher?** The Basic course is designed to establish certification and should be taken when: * No previous CITI training has been completed, or: * Prior CITI certification has been expired for a period greater than three years The Refresher course is designed to re-establish certification for three years and should be taken when: * The Basic course of a particular group has already been taken, and: * Recertification is required, but has not been expired for a period of three or more years Course required based on type of research: **Group 1: Biomedical Research** – Investigators conducting research about human biological systems and processes, including efficacy and safety of preventative, diagnostic or therapeutic methods must take this course. Types of research: * Clinical trial using a drug, medical device, technique or other intervention or strategy (including non-physical means, like diet, cognitive therapy, etc.) to diagnose, treat or otherwise study a particular condition or disease. * Non-clinical biomedical research to study normal or abnormal physical or physiologic processes (for example: gait and balance testing, biomechanical assessments, etc.). * Research involving medical records or data registries. * Research involving human biologic materials. **Group 2: Good Clinical Practice (GCP)** – Investigators conducting clinical trials funded by NIH, or utilizing an FDA regulated drug, device, or biologic must take this course. A clinical trial is defined as “a research study in which one or more human participants are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes”.Investigators conducting these types of trials must also take the Biomedical course (Group 1).
*This GCP course meets the minimum criteria for training identified by some sponsors. ([Check to see if your sponsor is listed](http://www.transceleratebiopharmainc.com/about/meet-the-members/).) If so, your CITI completion report can be supplied to the sponsor to meet their GCP training requirement.* **Group 3: Social & Behavioral** – Investigators conducting research performed with intent to study: * Behaviors, attitudes, and interactions/social processes among and between individuals, groups, and cultures. * Generally, this category of research has no intent of producing a diagnostic, preventative, or therapeutic benefit to the subject who is not seeking nor expecting a health benefit from the research. * This course is primarily taken by students at UNO, although it is common for Nursing Program students to be required to take this as well. ## Researchers/Students transferring from other institutions: Please email all completion reports for previously completed CITI training courses to [irbora@unmc.edu](mailto:irbora@unmc.edu). CITI courses are unique from institution to institution and transcript comparison will be required. Only the completion report shows the modules required for transcript comparison. Once previous training has been updated, the IRB will determine if any additional training or Refresher courses will be required.It is highly recommended that you email the Completion Report prior to beginning the Refresher course. Please do not send completion certificates.
## Collaborators with UNMC: Any independent entity collaborating with UNMC for the purposes of research must also complete CITI training as required by the institution. When registering for CITI, please affiliate with UNMC/UNO and do not register as an independent learner. If you need assistance with your username and/or password from an institution other than UNMC/UNO, you can contact: **CITI Support:** 888-529-5929 (9:00 a.m. to 7:00 p.m. EST/Monday – Friday). If you have more than one CITI account, you can request that they be merged by calling CITI Support. Once merged, all training completed will be available under one account. For all questions regarding CITI training, please contact the IRB Office at 402-559-6463 or email [irbora@unmc.edu](mailto:irbora@unmc.edu). # Community Partners All individuals that work on human subject research projects must complete **human subject research training**. Faculty, students, or employees of UNMC, NM, CHMC, UNO, BMC, or another academic partner institution are required to take CITI training. **Community partners who collaborate on research projects may complete the CIRTification program as an alternative to the CITI Program.** *** ## Who are Community Partners? Community partners are non-academic personnel (e.g. community leaders, representatives from supporting community organizations) engaged in research requiring IRB approval. This program is specifically and only for community members collaborating on human subject research studies. ## Who is NOT considered a Community Partner? Students or staff affiliated with UNMC, NM, CHMC, UNO, BMC, or research staff affiliated with other private entities directly engaging in human subject research. ## What is CIRTification? CIRTification is a training program in human research protections created by the University of Illinois Chicago CCTS that is tailored to the unique roles of community research partners. It is interactive and relevant to the roles and responsibilities that community partners have in research projects. The program considers community partners’ limited experience with research, discusses key concepts in research ethics and responsible conduct of research in plain language, and focuses on applying knowledge to real-life scenarios. Ideally, CIRTification Online will not only teach community partners about the importance of protecting research participants, it will also empower them to be active contributors to their respective research teams. CIRTification introduces learners to the basics of the research - the terminology, people, and methods. It reviews the history of research abuses that have informed current ethical principles, rules, and regulations. The training program also covers standard and best practices for: * Recruitment and informed consent * Collecting and protecting data * Handling changes that may arise during participant interactions * Reviews the role of the Institutional Review Board in protecting the right and safety of human research participants ## What can I expect? The program takes about 3-4 hours total to complete and can be completed in multiple sessions. The course is currently available in English, Spanish, and Haitian Creole. The course includes audio, video, text, and interactive activities. Learners will complete a knowledge quiz at the end of the program and receive a date-stamped certificate of completion. Please save a copy of this certificate for your records and email a copy to [IRBORA@unmc.edu](mailto:IRBORA@unmc.edu). ## Online Training Instructions: Please follow the instructions below to enroll in CIRTification. Please select **University of Nebraska Medical Center** only from the list. 1. Go to [https://training.ccts.uic.edu/](https://training.ccts.uic.edu/) 2. Click “Register” in the top right-hand corner. 3. Select “I am not from UIC”. 4. Complete the registration form. Under “Site”, select “University of Nebraska Medical Center”. 5. Once the form is filled in, click “Register” at the bottom of the form. 6. Visit the Course Catalog. Information about CIRTification will appear. Click “Learn More”. 7. Click “Enroll” to start the CIRTification course. ## Frequently Asked Questions (FAQ): |Who can I contact for help?| |:-| |For assistance, place contact: Megan Berger at [mberger@unmc.edu](mberger@unmc.edu)| |How long is this training valid?| |:-| |The training is valid for three years.| |How do I access my completion certificate?| |:-| |After completing the quiz at the end of the training, the option to print and save a date-stamped certificate of completion is available. If for some reason you missed this or are unable to complete this step, Megan Berger can access the completed training certificate. Email her at [mberger@unmc.edu](mberger@unmc.edu).| *CIRTification is funded through the University of Illinois at Chicago Center for Clinical and Translational Science and supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002003. We encourage mention and citation of CIRTification Online in grant proposals, conference presentations, published manuscripts, and other reporting. Suggested citation: CIRTification Online: Community Involvement in Research Training. University of Illinois at Chicago, Center for Clinical and Translational Science.* # Consent Forms ## Capacity to consent * [Assessment of capacity to consent to participate in research](https://guides.unmc.edu/attachments/196) ## Consent Form Readability Recent changes to the Federal Regulations governing human subject research (the “Common Rule”) have included a focus on improving the readability of consent forms and include regulations requiring understandable language, and organization and presentation of information that facilitates understanding. In response to these requirements, beginning October 31, 2019, consent forms must satisfy minimum readability standards. **We are working on a way to receive proof of PRISM training. Therefore, until further notice, we are not requiring physical proof, but written assurance.** Though we expect to extend the standard to other sections, initially only the readability of the **Invitation and Summary section** will be assessed. This section must have Flesch Kincaid reading level ≤8 and Flesch Reading Ease ≥60. Readability may be scored within the RSS application by clicking on the “Readability” button. CFs not meeting these minimum readability measures will be returned to the investigator for modification. To assist investigators and their staff in developing necessary skills to write effective consent forms, the PI and the person responsible for writing the consent form, **must complete online training through [PRISM](https://prism.kpwashingtonresearch.org/)** (Program for Readability in Science and Medicine). The hour-long training covers health literacy and readability, plain language strategies and examples, and interactive editing examples and exercises. *For more information on process, readability standards, and readability tips, see below.* **Readability Assessment Process:** * Readability can be assessed within the RSS application by clicking on the “Readability” button. This will open a new page with assessments of Flesch-Kincaid reading level and Flesch Reading Ease for the invitation and summary section. * In computing reading levels, technical terms (especially drug names and names of medical procedures) may unavoidably adversely affect the score. You may choose to use the name once, and then say "the test drug" or "the operation" subsequently. However, in all cases, an effort should be made to use a simpler word or phrase. * The IRB recognizes that sections of some consent forms may be of such a technical nature that it may not be possible to keep to an 8th grade reading level. In these situations, the invitation and summary at least must meet the standard described above, and the investigator must describe what additional tactics will be used to assure and assess comprehension. **Readability Standards:** * The Flesch–Kincaid readability tests are readability tests designed to indicate how difficult a passage in English is to understand. There are two tests, the Flesch Reading Ease, and the Flesch–Kincaid Grade Level. Although they use the same core measures (word length and sentence length), they have different weighting factors. * The results of the two tests correlate inversely: a text with a comparatively high score on the Reading Ease test should have a lower score on the Grade-Level test. * Many other measures of readability exist. SMOG (Simple Measure Of Gobbledygook) is a more exacting measure of readability, and accurately scores for the grade level required for complete text comprehension. (The Flesch-Kincaid formula grades for less than complete comprehension). SMOG demonstrates strong correlation with the required reading level in validation studies. The SMOG formula is based on the number of words consisting of three or more syllables. The SMOG score is commonly used to assess readability of healthcare information. **Tips for writing readable Consent Forms:** * PRISM online training is a web-based plain language tutorial for research professionals, including scientists, research staff, Institutional Review Boards (IRBs), or communications staff. The hour-long training covers health literacy and readability, plain language strategies and examples, and interactive editing examples and exercises. * There are a variety of tools available to assist investigators in improving readability of CFs. PRISM online training addresses some tactics. [The PRISM Readability Toolkit is available here](https://guides.unmc.edu/attachments/78). * The Readability Toolkit is a plain language handbook illustrating how to improve the readability of research consent forms and other materials for study participants. The Toolkit includes plain language principles and strategies, quick reference guide and editing checklist, Plain language alternatives to complex terms, easy-to-read template language for consent forms and links to readability resources. |***Do***|***Do Not***| |:-|:-| |Use short sentences and use words familiar to the non-medical reader.|Use medical terminology without explaining it, or use words that an 8th grader would not understand.| |Refer to thesauruses and medical glossaries made for children to find alternative ways to refer to medical terminology.|Use medical “jargon” or words longer than three syllables, when another word is also appropriate.| |Use the second person (“you”) and note that they are asked to participate in a research study. Be personal.|Use the third person (“the subject”), and avoid writing “invite” to refer to their participation.| |Use pictures and graphs wherever possible.|Provide information solely in large blocks of text, with long sentences.| |Say “for example” or “so forth.”|Say e.g. or etc.| |Use tablespoons or teaspoons to refer to the measurement of bodily fluids, and spell them out.|Use ml or cc to refer to volumes of bodily fluids, or abbreviate teaspoons/tablespoons.| |Say “greater than” or “less than”.|Use “>” or “<” or other symbols that an 8th grader might have trouble understanding at first glance.| |Describe study terminology such as “randomized”, “placebo”, or “double blind”; such as “like the flip of a coin”.|Use medical or study terminology without explaining it in lay terms.| |Use the words “study drug” or “study regimen”.|Use the terms “therapy” or “treatment” to describe drugs, devices, or procedures.| |Refer to investigational drugs or devices as “experimental” or “investigational”, and that it means the FDA has not yet approved it.|Refer to investigational drugs or devices as “new.”| **Readability Examples and Templates:** * [Invitation and Summary Template](https://guides.unmc.edu/attachments/79) * [Invitation and Summary Sample 1 (Phase I Oncology Drug)](https://guides.unmc.edu/attachments/80) * [Invitation and Summary Sample 2 (QOL Oncology)](https://guides.unmc.edu/attachments/81) * [Invitation and Summary Sample 3 (Phase III Drug)](https://guides.unmc.edu/attachments/82) * [Invitation and Summary Sample 4 (Knee Replacement)](https://guides.unmc.edu/attachments/83) ## Consent Teach-Back Tool UNMC is recommending that study teams utilize the "teach-back" method during the informed consent process for their clinical studies. This method will enhance the informed consent process, ensuring participants are well-prepared before consenting and enrolling in clinical studies. Our aim is to empower clinical research investigators and coordinators with the tools and knowledge to ensure that research study participants are fully informed and understand all aspects of the informed consent form. [**Teach-Back Tool**](https://guides.unmc.edu/attachments/84) **Ethical Responsibility in Research** * Disclosure: It's our duty to provide all necessary information to potential research participants. * Decision-making: We must ensure that participants can make informed decisions based on the information provided. **The Importance of Health Literacy** "Health literacy plays a crucial role in maintaining a healthy lifestyle and making informed decisions about our health care. Yet, a report from the HHS Surgeon General in 2019 highlighted that *only 12% of Americans possess proficient health literacy skills*. This underscores the importance of clear communication in the informed consent process." *Reference: HHS Surgeon General Reports and Publications, 2019* **Consent Teach Back: A Strategy for Clarity** * A strategy to improve the researcher’s ability to explain the ICD content in a way that is clear and understandable. * An opportunity to facilitate understanding of why a participant may or may not want to participate. * A tool to assure that the participant is provided with sufficient opportunity to discuss the information given to them and to consider whether or not to participate **The 5 T’s of Teach Back** 1. Triage: Concentrate on one topic at a time. 2. Tools: Utilize models, written tools, posters, graphics, etc., to aid in explaining the desired information. 3. Take Responsibility: Phrase it as, “I want to make sure I did a good job explaining…” 4. Tell Me: Encourage the participant to express their understanding in their own words. Be specific about what you expect them to relay back. 5. Try Again: If the participant's understanding isn't clear, revisit the topic. *Reference: Anderson, Leister and DeRego, Health Literacy Research and Practice, 2020* **Evaluating Understanding with Teach-Back** * Participant’s Understanding of the Study: * Purpose * Procedures * Risks * Appreciating the Consequences of Participation: * Recognizing it's a research study * Potential benefits (or lack thereof) * Impact on current or future treatments * Confidentiality and data access * Participant’s Reasoning/Decision Process: * Awareness of other options * Understanding that participation is VOLUNTARY * Participant’s Ability to Make a Choice. # E-Signature Instructions The e-signature function in RSS is a way to electronically sign consent forms. It can be used if the consent process is conducted in person (face to face) or remotely (telephone or video as approved by the IRB). RSS e-signature can only be used for certain studies, outlined in the table below. *E-signature can only be used on newly created Narrative Consent Forms.* **When can RSS e-signature be used? (as of 2/20/2024)** |Study Types| | |:-|-| |FDA-regulated (drug/device) studies|Yes| |Non-FDA-regulated studies|Yes| |Commercially funded studies|Yes| |Federally funded studies|Yes| |CIRB (studies relying on an external IRB)|**No**| |Multi-site studies where UNMC is the IRB of record|**No**| Consent forms that are electronically signed in RSS are maintained in the RSS application, so paper forms do not need to be printed and stored. See the links below for instructions on in person or remote e-signatures: * [E-signature for in person consent](https://guides.unmc.edu/attachments/255) * [E-signature for remote consent](https://guides.unmc.edu/attachments/254) If you have any questions or would like to schedule a training session, please contact Sue Logsdon at [slogsdon@unmc.edu](slogsdon@unmc.edu). # IRB Training Videos Below are training topics related to the IRB, its history, and its function. Please let these videos serve as a training tool for those new to the IRB and research fields. If you need individual assistance with specific issues, or for general information, please contact us at [irbora@unmc.edu](mailto:irbora@unmc.edu) or through the RSS Message Portal. ### [Basics: Introduction to the Institutional Review Board (2021)](https://echo360.org/media/c7a9e2c5-a9bc-4bc9-a606-b0ce5ca049a5/public) ### [Basics: New to Human Subjects Research? (5/5/2025)](https://echo360.org/media/570267b8-34d7-469b-9192-5b3e4cf12c9d/public) ### [Ethical Access to Patients as Human Subjects of Research (2021)](https://echo360.org/media/49c924a3-8e96-46e5-9339-c1d989907b4f/public) ### [Getting Approved by the IRB: Everything You Need to Know (2021)](https://echo360.org/media/3734cd59-ee02-441a-99b7-8356775c0fba/public) ### [Human Subject Research vs Not Human Subject Research (5/8/2025)](https://echo360.org/media/cfe5de6a-f9f7-4790-97cc-58c264945bd9/public) ### [Informed Consent (2021)](https://echo360.org/media/e3deb000-44cd-4afa-a356-454197f2686d/public) ### [IRB Considerations in Research Involving Drugs and Devices (2021)](https://echo360.org/media/800bddd3-9fd6-47be-96db-a2d75792eb28/public) ### [Use of the IRB Short Form (2021)](https://echo360.org/media/6a3fec4f-1b78-4603-b6bf-7b6408f0c4b8/public) # RSS Training **Guides to assist with RSS procedures:** If there is a topic that does not appear below, please contact [irbora@unmc.edu](mailto:irbora@unmc.edu) *** #### [Adding Personnel](https://guides.unmc.edu/attachments/258) #### [Adding Documents to an Application](https://guides.unmc.edu/attachments/15) #### [Consent Forms 101](https://guides.unmc.edu/attachments/94) #### [Creating and navigating a New Application](https://guides.unmc.edu/attachments/93) #### [Deleting Personnel](https://guides.unmc.edu/attachments/259) #### [Functions within a Consent Form](https://guides.unmc.edu/attachments/91) #### [How to submit an Incident Report](https://guides.unmc.edu/attachments/92) #### [Navigating the RSS Dashboard](https://guides.unmc.edu/attachments/90) #### [Signatures on a Consent Form](https://guides.unmc.edu/attachments/89) #### [Submitting a Change Request](https://guides.unmc.edu/attachments/88) #### [Using the in-person RSS E-Signature](https://guides.unmc.edu/attachments/201) #### [Using the remote RSS E-Signature](https://guides.unmc.edu/attachments/200) #### [Working within an Application 101](https://guides.unmc.edu/attachments/87) # Virtual Training/Office Hours **IRB Office Hours** We can be reached by phone (402-559-6463) or by email ([irbora@unmc.edu](mailto:irbora@unmc.edu)) Mon-Fri 7am-5pm. The IRB offers two virtual learning/training sessions each month. * 2nd Monday of every month 9-10 AM ([Zoom link](https://unmc.zoom.us/j/97863988844?pwd=OUxlRGFpQ2JBQWRwcngxdGU0OWk3Zz09)) * 4th Thursday of every month 2-3 PM ([Zoom link](https://unmc.zoom.us/j/97863988844?pwd=OUxlRGFpQ2JBQWRwcngxdGU0OWk3Zz09)) Please direct questions regarding virtual training hours to the IRB Education Coordinator: Megan Berger [mberger@unmc.edu](mberger@unmc.edu) or 402-559-6044 # Forms The following sections (located in the menu below will assist researchers by providing various tools and resources.The IRB uses an electronic IRB submission system referred to as RSS. Never used the system before? Check the RSS Training section to learn more.The IRB's Policy & Procedures Manual is available in UNMC Guides - https://guides.unmc.edu/books/hrpp-policies-and-procedures # Existing paper protocolsNOTE: THE PAPER FORMS ON THIS PAGE ARE ONLY TO BE USED FOR STUDIES THAT HAVE NOT BEEN TRANSFERRED TO THE ELECTRONIC SYSTEM (I.E. THEY ARE STILL IN PAPER FORMAT).
## FORMS FOR EXISTING PAPER PROTOCOLS ONLY |**Title**|**Version**|**Format**| |:-|:-|:-| |1. CR Biomedical Research|06/13/2022|[Word](https://guides.unmc.edu/attachments/101)| |2. CR Social and Behavioral Research|06/13/2022|[Word](https://guides.unmc.edu/attachments/96)| |3. CR Human Biological Materials|06/13/2022|[Word](https://guides.unmc.edu/attachments/95)| |4. Study Completion Report|07/27/2010|[Word](https://guides.unmc.edu/attachments/97)| |5. Request for Change in Protocol (*Depending on the nature of the change completion of a protocol addendum may be required. Please contact the ORA for details.*)|03/10/2014|[Word](https://guides.unmc.edu/attachments/98)| |6. Request for Change* (Ads, Educational Items and Personnel Only)|05/14/2013|[Word](https://guides.unmc.edu/attachments/99)| |7. Additional Personnel Change Form Pages (if more than four spaces needed)|08/17/2010|[Word](https://guides.unmc.edu/attachments/100)| |8. P&T Drug Registry Form| 04/14/2022|[Word](https://guides.unmc.edu/attachments/191)| # FWAs - Federal Wide Assurance The UNMC IRB Federal Wide Assurance documents can be found here: ### [Adult IRB Roster -2025 (FWA00002939)](https://guides.unmc.edu/attachments/260) ### [Pediatric IRB Roster -2025 (FWA00002939)](https://guides.unmc.edu/attachments/261) # Miscellaneous |**Title**|**Version**|**Format**| |:-|:-|:-| |Investigator Assessment Checklist for Regulatory Documentation|9/17/2007|[Word](https://guides.unmc.edu/attachments/194)| |Investigator Assessment of IRB|9/7/2007|[Word](https://guides.unmc.edu/attachments/193)| # Recruitment templates HRPP policy to review: [#3.5 Subject Recruitment Through Advertisements](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/35-subject-recruitment-through-advertisements) |**Recruitment Type**|**Format**| |:-|:-| |Flier - full page|[Word](https://guides.unmc.edu/attachments/104)| |Ad - one column|[Word](https://guides.unmc.edu/attachments/107)| |Ad - two column|[Word](https://guides.unmc.edu/attachments/106)| |Ad - six inch|[Word](https://guides.unmc.edu/attachments/105)| # Short Forms A Short Form may be used when a subject/LAR who cannot understand English is unexpectedly encountered, there is not sufficient time to develop and obtain IRB approval for a complete ICF written in language understandable to the subject/LAR, AND the research presents the prospect of direct therapeutic benefit to the subject. Use of the Short Form is limited to enrollment of no more than three subjects per language in a given protocol. In order to enroll more than three subjects, the PI is required to have the complete ICF translated into the appropriate language and reviewed and approved by the IRB. The investigator must complete a Short Form Request (available in RSS) for each subject, and submit to the ORA. Each request must be approved before the Short Form may be used. Depending on the nature and duration of the research, the IRB may determine that the English version of the complete ICF must be translated into a language understandable to the subject and a copy given to the subject as soon as possible after enrollment. In general, this may be required for studies which are significant risk and of long duration. Please refer to [HRPP Policy 5.5](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/55-use-of-the-short-form-consent-document) (Use of the Short Form Consent Document) for detailed instructions.English translations of these short forms is included at the bottom of the page. Translated Short Forms generously supplied by Office of Human Subjects Research - Johns Hopkins Medicine. (Key: * - Johns Hopkins University)
*** ### Short Forms [Albanian Short Form *](https://guides.unmc.edu/attachments/265) [Arabic Short Form *](https://guides.unmc.edu/attachments/266) [Burmese Short Form *](https://guides.unmc.edu/attachments/267) [Cantonese Short Form *](https://guides.unmc.edu/attachments/268) [Chinese Short Form *](https://guides.unmc.edu/attachments/269) [English Translation *](https://guides.unmc.edu/attachments/264) [Farsi Short Form *](https://guides.unmc.edu/attachments/270) [French Short Form *](https://guides.unmc.edu/attachments/271) [Greek Short Form *](https://guides.unmc.edu/attachments/272) [Gujarati Short Form *](https://guides.unmc.edu/attachments/273) [Haitian Creole Short Form *](https://guides.unmc.edu/attachments/274) [Hebrew Short Form *](https://guides.unmc.edu/attachments/275) [Hindi Short Form *](https://guides.unmc.edu/attachments/276) [Hungarian Short Form *](https://guides.unmc.edu/attachments/277) [Italian Short Form *](https://guides.unmc.edu/attachments/278) [Japanese Short Form *](https://guides.unmc.edu/attachments/279) [Karen Short Form (LLS)](https://guides.unmc.edu/attachments/280) * [Karen Certificate of Translation](https://guides.unmc.edu/attachments/263) [Korean Short Form *](https://guides.unmc.edu/attachments/281) [Lao Short Form *](https://guides.unmc.edu/attachments/282) [Luganda Short Form *](https://guides.unmc.edu/attachments/283) [Mandarin Short Form *](https://guides.unmc.edu/attachments/284) [Nepali Short Form *](https://guides.unmc.edu/attachments/285) [Polish Short Form *](https://guides.unmc.edu/attachments/286) [Portuguese Short Form *](https://guides.unmc.edu/attachments/287) [Romanian Short Form *](https://guides.unmc.edu/attachments/288) [Russian Short Form *](https://guides.unmc.edu/attachments/289) [Somali Short Form *](https://guides.unmc.edu/attachments/290) [Spanish Short Form *](https://guides.unmc.edu/attachments/291) [Swahili Short Form *](https://guides.unmc.edu/attachments/292) [Thai Short Form *](https://guides.unmc.edu/attachments/293) [Ukranian Short Form *](https://guides.unmc.edu/attachments/294) [Urdu Short Form *](https://guides.unmc.edu/attachments/295) [Vietnamese Short Form *](https://guides.unmc.edu/attachments/296) *Translated Short Forms generously supplied by Office of Human Subjects Research - Johns Hopkins Medicine (Key: * - Johns Hopkins University)* # Subject's rights & responsibilities ## Research Questions: [Amheric - Research Questions & Rights and Responsibilities](https://guides.unmc.edu/attachments/250) [English (UNMC)](https://guides.unmc.edu/attachments/170) [English (UNO)](https://guides.unmc.edu/attachments/172) [Spanish (UNMC)](https://guides.unmc.edu/attachments/171) [Spanish (UNO)](https://guides.unmc.edu/attachments/173) ## Rights of Research Subjects: [English](https://guides.unmc.edu/attachments/174) [English (CHMC)](https://guides.unmc.edu/attachments/178) [French](https://guides.unmc.edu/attachments/175) [Somali](https://guides.unmc.edu/attachments/176) [Spanish](https://guides.unmc.edu/attachments/177) ## Rights and What to know: [French](https://guides.unmc.edu/attachments/179) [Karen](https://guides.unmc.edu/attachments/180) ## Interpretation Certificates: [Certificate 1](https://guides.unmc.edu/attachments/181) [Certificate 2](https://guides.unmc.edu/attachments/182) # Single & Central IRB RSS For applications with due dates on or after Jan 25, 2018, and contract solicitations published on or after Jan 25, 2018, NIH expects that all sites participating in multi-site studies, which involve non-exempt Human Subjects Research(HSR) funded by the NIH, will use a single Institutional Review Board(sIRB) to conduct the ethical review required for the protection of human subjects.This policy applies to the domestic sites of NIH-funded multi-site studies where each site will conduct the same protocol involving non-exempt HSR. It does not apply to career development, research training or fellowship awards. Implementation of the NIH sIRB policy is expected to reduce unnecessary administrative burdens and systemic inefficiencies while maintaining appropriate human subjects protections._________________________________________________________________________________________________________The UNMC sIRB team serves as resource for UNMC and external site study teams collaborating on multi-site projects.Our mission is to provide high quality, timely IRB review for multi-site HSR.Please speak with an sIRB analyst prior to submitting an sIRB request. Any questions regarding applications, fee schedules, consultations, and more please contact the sIRB team at sirb@unmc.edu._________________________________________________________________________________________________________For multi-center studies, the central IRB (cIRB) is the IRB that conducts reviews on behalf of all study sites that agree to participate in the centralized review process. For sites at institutions that have an IRB that would ordinarily review research conducted at the site, the cIRB should reach agreement with the individual institutions participating in centralized review and those institutions' IRBs about how to apportion the review responsibilities between local IRBs and the cIRB (21 CFR 56.114). # Glossary ##### Cede Review An institution agrees to transfer IRB review and oversight authority for specified research to another institution’s IRB (reviewing IRB). ##### cIRB <<<< insert definition >>> ##### Lead Site <<<< insert definition >>> ##### Lead Principal Investigator (Lead Site PI) The study wide lead Principal Investigator with ultimate responsibility for the conduct and integrity of multisite research. ##### Local Context Unique legal requirements, cultural or religious values, or other site-specific variables that exist at a site where subjects are enrolled in research. ##### Participating Site (pSite) <<<< insert definition >>> ##### Participating Site Principal Investigator (pSite PI) The lead investigator at each institution participating in multisite research usually responsible for the conduct of the research at the participating institution. ##### Reliance <<<< insert definition >>> ##### Reliance Agreement (also known as an Authorization Agreement) An agreement between two Organizations engaged in human subject research that documents respective authorities, roles, responsibilities, and communication between the reviewing and relying IRBs. ##### Reliance Negotiation <<<< insert definition >>> ##### Relying Institution A participating Institution that cedes IRB review to the IRB of record (reviewing IRB) designated under a Reliance Agreement. ##### Relying IRB <<<< insert definition >>> ##### Reviewing IRB The IRB which is responsible for conducting IRB review and approval as described in 45 CFR 46.109 for cooperative human subject research. ##### sIRB <<<< insert definition >>> # sIRB - What is Single IRB? Single IRB is a model of IRB review that is intended to streamline and reduce administrative burden on IRBs reviewing multisite studies. In a single IRB model, one IRB serves as the reviewing IRB or “IRB of Record” and the participating site (pSite) IRBs serve as relying IRBs. The responsibilities of each IRB are outlined in a document called a reliance agreement. *For more information on the history of single IRB (sIRB) review and sIRB review at UNMC, see each section below:* ## Single Site Research: Below is a model of single site research, or research that is only taking place at one site. [](https://guides.unmc.edu/uploads/images/gallery/2024-08/3ukM8IdcLGUi3wcu-single_site_research.png) ## Multi-site Research: Historically, multisite research was overseen by multiple independent IRBs. This often led to differences in IRB review and outcomes across sites. Below is a model of historical multisite research, or research that took place at more than one site. [](https://guides.unmc.edu/uploads/images/gallery/2024-08/KAjq6OqTpaVbX7xw-multi_site_research.png) ## Single IRB Review Requirement: The requirement for single IRB review originated from the NIH single IRB policy and the Revised Common Rule. **From the NIH:** "expectation that all [domestic] sites participating in multi-site studies involving non-exempt human subjects research funded by the National Institutes of Health will use a single Institutional Review Board (sIRB)" (NOT-OD-16-094, June 2016) * effective date January 25, 2018 For more information on the NIH Single IRB Policy visit: [https://grants.nih.gov/policy/humansubjects/single-irb-policy-multi-site-research.htm.](https://grants.nih.gov/policy/humansubjects/single-irb-policy-multi-site-research.htm) For Guidance on exceptions to the NIH Single IRB Policy, visit: [https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-003.html](https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-003.html) **From the Revised Common Rule:** "Any institution located in the United States that is engaged in cooperative research must rely upon approval by a single IRB …" (§_.114(b)(1)) * effective date January 20, 2020 For more information on the Revised Common Rule's Single IRB Requirement, visit: [https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46#46.114.](https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46#46.114) For a list of Common Rule Departments and Agencies, visit: [https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html](https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html) For information on Common Rule Exception Determinations, visit: [https://www.hhs.gov/ohrp/regulations-and-policy/single-irb-requirement/index.html#exceptions](https://www.hhs.gov/ohrp/regulations-and-policy/single-irb-requirement/index.html) In short, this means that studies that are federally funded and taking place at more than one site are now required to use a single IRB as the IRB of record. There are many circumstances where it is not clear if a specific study requires single IRB review or if an exception may apply. In these cases, please email [sirb@unmc.edu](mailto:sirb@unmc.edu) for assistance at the earliest opportunity.Note: Participating site IRBs are still involved in the single IRB process and have their own local requirements, but the responsibility for the regulatory component of review is ceded to the single IRB. Each IRB’s responsibilities are outlined in a document called a Reliance Agreement. More information on reliance can be found in the sIRB Reliance section below.
## Single IRB Review Models: Some groups were already utilizing a single IRB model prior to the requirement (e.g., commercial IRBs). Below is a simplified model of how some commercial IRBs handle multisite research. Many will interact directly with the sponsor, perform IRB review activities, and then push approvals to all sites simultaneously. [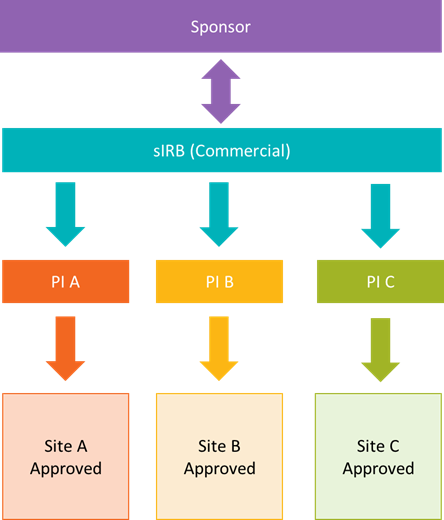](https://guides.unmc.edu/uploads/images/gallery/2024-08/UhC6ptTumx0a9jgW-commercial_irb_1.png) Below is another model of how a single IRB may handle multisite research. In this example, the sponsor works with each site PI and study team independently. Each site submits their own application to the single IRB for review and receives separate approvals. [](https://guides.unmc.edu/uploads/images/gallery/2024-08/UhC6ptTumx0a9jgW-commercial_irb_1.png) ## sIRB Review at UNMC: Single IRB Review at UNMC is broken out into two main parts. First, the overall study and lead site-specific requirements are reviewed. After overall study and lead site approval, participating sites (pSites) are onboarded and approved on a rolling basis. [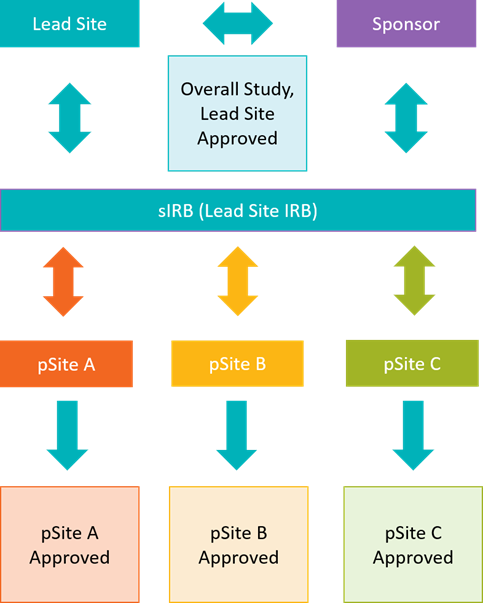](https://guides.unmc.edu/uploads/images/gallery/2024-08/hQN8yIubtY0wP0p7-sirb_review_unmc.png)Note: pSite IRB processes are not included in the above models. Please contact your pSite IRB directly with questions.
# sIRB - UNMC process The UNMC sIRB Process is captured in the diagram below. [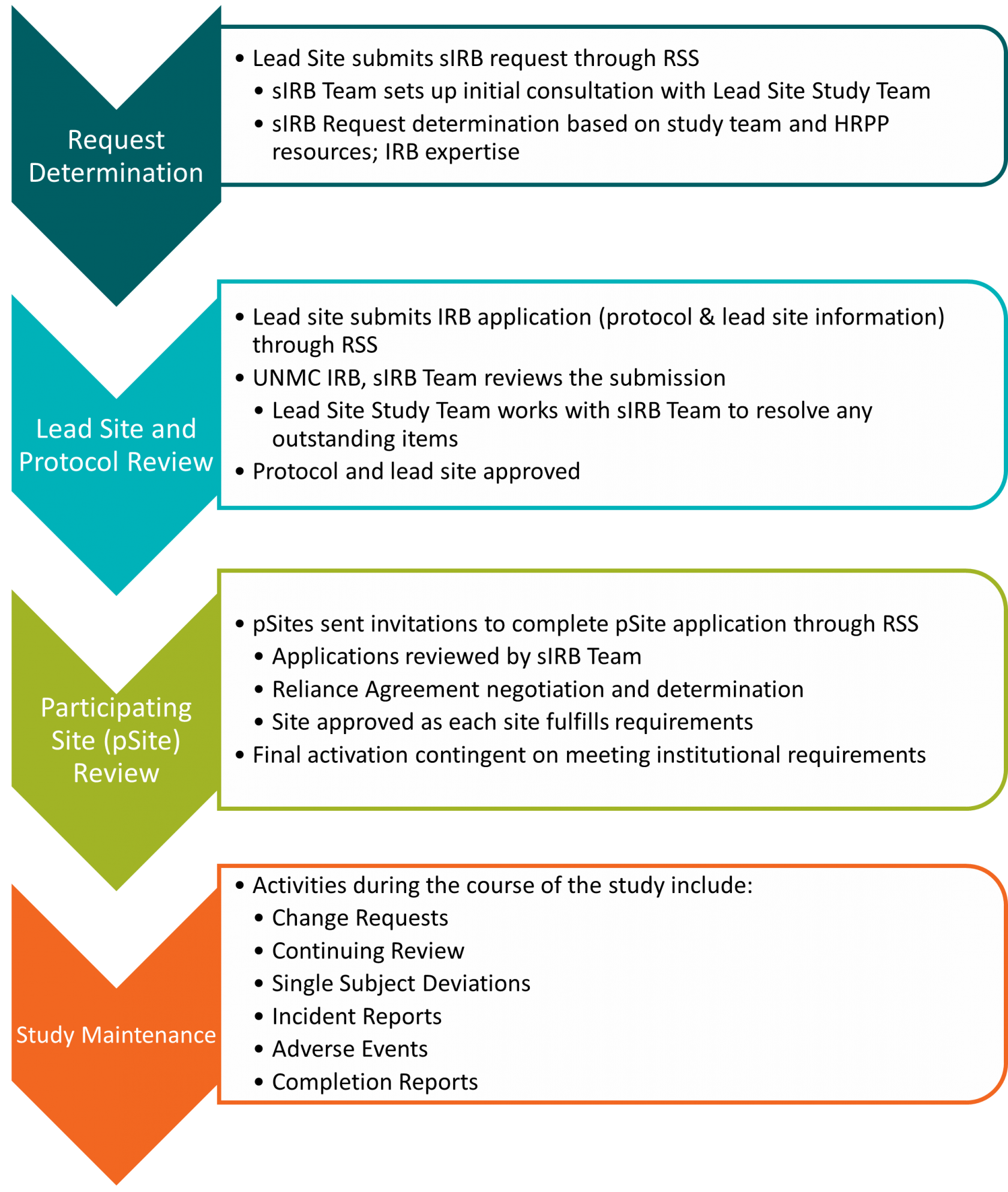](https://guides.unmc.edu/uploads/images/gallery/2024-08/nmUnC9ukVUn3wlPw-sirb_process.png) Contact [sirb@unmc.edu](mailto:sirb@unmc.edu) with any UNMC sIRB process questions. # sIRB - Submission deadlines ## sIRB Submission Deadlines for New Submissions, Previously Tabled Protocols and Requests for Change * Deadline for Continuing Review submission is the 1st day of the month prior to expiration. * [Full Board Deadlines (printable PDF)](https://guides.unmc.edu/attachments/257) *** ### sIRB Full Board Submission Deadline – 9 am | ***Submission Deadline*** | ***Date of sIRB Meeting*** | |---------------------|-----------------------| |2/6/2025|2/14/2025| |3/6/2025|3/14/2025| |4/3/2025|4/11/2025| |5/1/2025|5/9/2025| |6/5/2025|6/13/2025| |7/3/2025|7/11/2025| |7/31/2025|8/8/2025| |9/4/2025|9/12/2025| |10/2/2025|10/10/2025| |11/6/2025|11/14/2025| |12/4/2025|12/12/2025| |12/31/2025|1/9/2026| # sIRB - Reliance Reliance refers to an arrangement where an IRB allows another IRB to perform review and approval of a study. The UNMC IRB uses the term “single IRB” or “sIRB” to indicate that UNMC is serving as the reviewing IRB (i.e., the IRB of record) of a multisite study. ## Reliance Agreement A reliance agreement is a formal, written document that provides a mechanism for an institution engaged in research to delegate IRB review to another IRB. This document generally outlines the responsibility of the single IRB and the participating site. Often the reliance agreement is accompanied by addenda or other forms that are designed to further clarify responsibilities or collect pSite local context information. Reliance agreements come in many formats. They may also be called: * IRB Authorization Agreement (IAA) * “Cooperative,” “Network,” or “Master” Agreement * Reliance Memorandum of Understanding (MoU) * Institutional Investigator Agreement (IIA) * Letter of Acknowledgement, “Cede” Letter ## UNMC's Preferred Reliance Agreement Format UNMC is a [SMART IRB](https://smartirb.org/) participating site and *strongly* prefers to document reliance using the [SMART IRB Online Reliance System](https://smartirb.org/reliance/) (SMART IRB ORS). In addition, we ask that sites complete the SMART IRB Implementation Checklist. This will be provided during the reliance negotiation.Note: SMART IRB is not an IRB, but a tool that IRBs use to document reliance.
Note: The UNMC sIRB Team has elected to initiate and maintain requests in the SMART IRB ORS, rather than have the lead site investigator or study team create requests. Please contact sirb@unmc.edu with questions.
If a site is not a SMART IRB participating institution, other reliance formats will be made available for use during the reliance negotiation. ## Reliance Negotiation The term reliance negotiation refers to the process of the reviewing and relying IRB representatives communicating and agreeing to the reliance agreement format and terms. The UNMC sIRB Team will initiate the negotiation during the pSite Onboarding stage of review. # sIRB - Fees **For information regarding the fee schedule for Single IRB studies, please contact [sirb@unmc.edu](mailto:sirb@unmc.edu).** # cIRB - What is Central IRB? The UNMC IRB uses the term “central IRB” or “cIRB” to indicate that the reviewing IRB (i.e., the IRB of record) of a multisite study is external to UNMC. The UNMC IRB has contracted with two commercial IRBs, Advarra and WCG. In addition, the UNMC IRB partners with consortium, academic, and medical center IRBs nationwide. For more details around the use of a cIRB, including when the use of an external central IRB is not permitted, see UNMC [HRPP Policy 1.4](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/14-unmc-ceding-review-to-an-external-central-irb) (UNMC Ceding Review to an External Central IRB). # cIRB - UNMC ProcessNote: When requesting cIRB review, study teams must satisfy the requirements of each IRB and the funding agency or sponsor (as applicable), prior to initiating the research.
## UNMC cIRB Initial Review Process [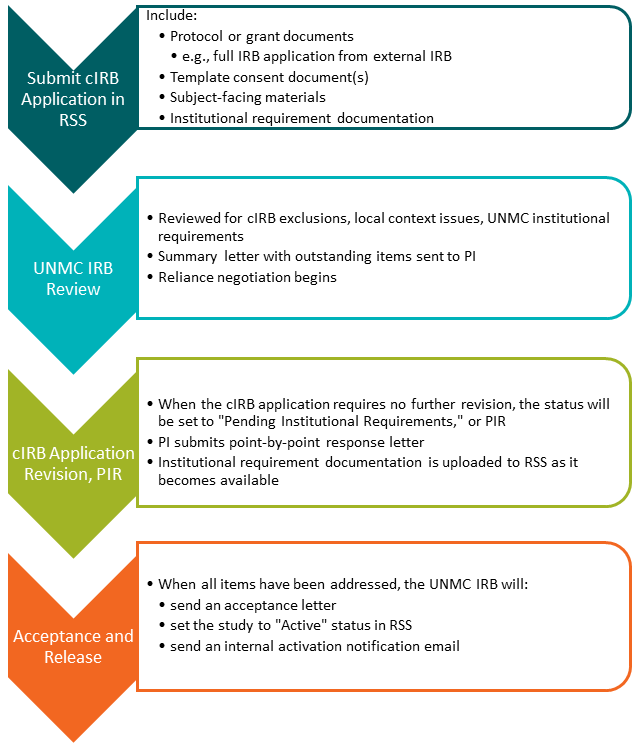](https://guides.unmc.edu/uploads/images/gallery/2024-08/8ODP07bDyQa6SHPp-cirb_review_process.png) ## External cIRB Process * Contact your external cIRB representative for details. Each IRB operates differently. * IRB approval is needed prior to initiating research ## Funding Agency/ Sponsor Process * Contact your funding agency/sponsor representative for details. Each group operates differently. * Funding agency/sponsor approval is needed prior to initiating research ## Post-acceptance Study Activities In general, the external IRB of record will be responsible for reviewing most study activities. In some cases, these may also need to be submitted to the UNMC IRB. Things to submit to the **external cIRB**: * Amendments * Continuing Reviews * Single-subject Deviation Requests * Short-form Requests * Incident Reports * Adverse Event Reports * Study Completion ReportsNote: In most cases, submit to the external IRB. If determined to be necessary, the external cIRB will notify the UNMC IRB directly or request the study team notify the UNMC IRB.
## Things to submit to the UNMC IRB: * New or modified Conflicts of Interest, management plans or additional external cIRB requirements * Copies of reports made to OHRP and/or FDA * Copies of Incident Reports submitted to the cIRB * Copies of Internal Adverse Event Reports submitted to the cIRB * Personnel ChangesNote: personnel are NOT permitted to work on a study until UNMC IRB approval is received.
* Annual Status Update via Demographic Recruiting Numbers Form * Study Closure NotificationsNote: Send a notification in the message portal, indicating the study is now closed. Upload applicable documentation.
# cIRB - Reliance When requesting to rely on an external IRB, the UNMC IRB will assess the external IRB’s qualifications in accordance with [HRPP Policy 1.4](https://guides.unmc.edu/books/hrpp-policies-and-procedures/page/14-unmc-ceding-review-to-an-external-central-irb) (UNMC Ceding Review to an External Central IRB). The UNMC IRB may request information related, but not limited, to: * SMART IRB Participation * HRPP Accreditation or Completion of OHRP QA Self-Assessment Tool * Valid FWA, Registration with OHRP and FDA (as applicable) The UNMC IRB strongly prefers to document reliance using the SMART IRB Online Reliance System. UNMC has several reliance templates based on institution type available for use. Other reliance formats are approved on a case-by-case basis. [](https://smartirb.org/ "Visit Smart IRB") # cIRB - Fees In line with most other academic medical centers and universities, the Office of Regulatory Affairs and the IRB charges an initial submission and an annual continuing review fee for commercial and industry sponsored protocols. **Central IRB:** |*effective 7/1/2021*|**Initial Submission**|**Annual Continuing Reviews**| |:-|:-|:-| |Central IRB Review|$3000|N/A| **Commercial/Industry Sponsored Research Protocols:** |*effective 7/1/2021*|**Initial Submission**|**Annual Continuing Reviews**| |:-|:-|:-| |Full Board|$3000|$1250| |Expedited|$2000|$1000| **Center for Nutrition & Health Impact** |Please contact [sirb@unmc.edu](mailto:sirb@unmc.edu) for more information.| |-| **Single IRB:** |Please contact [sirb@unmc.edu](mailto:sirb@unmc.edu) for more information.| |-| # cIRB - Forms & LinksNote: the UNMC IRB cannot answer questions related to external IRB processes or applications. Contact the external IRB's helpdesk or designated representative directly for assistance.
For questions regarding UNMC cIRB processes or applications, please contact [sirb@unmc.edu](sirb@unmc.edu). ## Advarra * Advarra IRB Application System, CIRBI: [https://www.cirbi.net](https://www.cirbi.net/) * Approved UNMC - [Advarra Consent Local Context Information Document](https://guides.unmc.edu/attachments/183) (11/2/2023) * [Advarra Checklist](https://guides.unmc.edu/attachments/184) (optional) *Advarra has requested the UNMC IRB number be added to the application as follows:* * For single-site protocol applications: in the field below in the screen shot (with the x’s). [](https://guides.unmc.edu/uploads/images/gallery/2024-08/IFarMfWQ26J72e5o-single_study_example_1.png) * For site only (SSU) applications: add the internal number to the following area on the investigator application: [](https://guides.unmc.edu/uploads/images/gallery/2024-08/jLkjUxUqnZtCPcer-single_study_example_2.png) ## Contact Information Sheet The UNMC IRB recognizes that not all central IRB consent templates have the option to add local study contacts. The cIRB application in RSS now has the option to build a “Contact Information Sheet” to be provided to subjects along with the approved consent documents. ***Older studies within RSS may not have this functionality. The [Contact Information Sheet Template](https://guides.unmc.edu/attachments/185) may be downloaded for manual completion. ## Miscellaneous central IRBs * Contact the external IRB for information regarding their IRB review process and any applicable IRB application management systems. * [UNMC cIRB Local Context Information Document (11/2/2023)](https://guides.unmc.edu/attachments/186) ## NCI CIRB * NCI CIRB Application System, IRBManager: [https://nci.my.irbmanager.com/](https://nci.my.irbmanager.com/) * Approved [Univ of Nebraska Consent Local Context Information Document (9/7/2022)](https://guides.unmc.edu/attachments/187) ## NMDP * [UNMC NMDP HIPAA standalone (5/8/2025)](https://guides.unmc.edu/attachments/298) ## SMART IRB * SMART IRB [https://smartirb.org/](https://smartirb.org/) is not an IRB, but a tool used to document reliance between IRBs. * The IRB representatives from each institution will manage this negotiation. Unless otherwise indicated, nothing is needed from the investigator or study teams to fulfill this requirement. ## WCG * WCG IRB Application System, WCG IRB Connexus: [https://identity-connexus.wcgirb.com](https://identity-connexus.wcgirb.com/) * Approved U of Nebraska - [WCG Consent Local Context Information Document (1/12/2024)](https://guides.unmc.edu/attachments/189) If requesting revisions to the template consent language, the University of Nebraska – [WCG ICF Checklist](https://guides.unmc.edu/attachments/190) must be completed and signed by the study’s lead UNMC IRB representative. # SROC In accordance with all Federal Guidelines, the University of Nebraska and Board of Regents policy, the Scientific Research Oversight Committee (SROC) must review and approve all research using hESC lines and ONLY lines from the NIH Human Embryonic Stem Cell Registry (http://grants.nih.gov/stem_cells/registry/current.htm?sort=rnd) are allowed. Cell lines which are not on this registry are not allowed to be used for NU System research. The NIH Registration Number for a proposed stem cell line must be cited on your application for hESC use when submitting your application to the SROC. The committee must be composed of scientists who have hESC expertise and at least 2 non-affiliated members, one of which must be a non-scientist. Human embryonic stem cell research at UNMC: Current and prior protocols address a wide range of topics from the very basic mechanisms that regulate stem cell self renewal, which is the primary characteristic that distinguishes stem cells from all other cell types in the body, to potential applications of stem cells in regenerative medicine.Studies have demonstrated the derivation of human hepatocytes (liver cells) from hES cells, retinal and neuronal (eye and brain) cells and shown that stem cells that are differentiated to fibroblasts of the lung in conditions similar to those in patients with asthma, are much more contractile than normally derived lung fibroblasts. This has prompted a new hypothesis to explain the persistence of asthma even in the face of anti-inflammatory treatments. Additional projects address the education aspects of the properties of hES cells. These results have been published in nationally ranked and recognized medical journals. All studies must be SROC and IRB approved and employ federally approved hES cell lines that are used according to all federal, state and university regulations. # Chairs and Administrator [](https://guides.unmc.edu/uploads/images/gallery/2024-08/FMuzNT3qgqza7ZOf-ahmad.jpg) **Iqbal Ahmad, PhD** * Chair, SROC * 402-559-4091 * [Email](mailto:iahmad@unmc.edu) [](https://guides.unmc.edu/uploads/images/gallery/2024-08/NxPJdn6vCiAfTfYu-logsdon-sue.jpg) * IRB Analyst III, SROC * 402-559-3779 * [Email](mailto:slogsdon@unmc.edu) # Committee In 2008, the Human Embryonic Stem Cell Research Advisory Committee of the National Research Council and Institute of Medicine published [Amendments to the National Academies’ Guidelines for Human Embryonic Stem Cell Research](http://www.nap.edu/catalog/12260.html). Section 2.0 (page 11) describes the establishment of an Institutional ESCRO “To provide oversight of all issues related to derivation and use of hES (human embryonic stem) cell lines and to facilitate education of investigators involved in hES cell research, each institution should have activities involving hES cells overseen by an Embryonic Stem Cell Research Committee (ESCRO).” “An ESCRO committee should include independent representatives of the lay public as well as persons with expertise in developmental biology, stem cell research, molecular biology, assisted reproduction and ethical and legal issues in hES cell research.” (Since in Nebraska, development of human stem cell lines is not permitted, assisted reproduction input is potentially less relevant than in states that do not have this prohibition.) “It must have suitable scientific, medical and ethical expertise to conduct its own review and should have the resources needed to coordinate the management of various other reviews required for a particular protocol.” (eg animal use, recombinant DNA, human subject research) “But the ESCRO committee should not be a subcommittee of the IRB, as its responsibilities extend beyond human subject research.” (For example, in Nebraska, this includes ensuring that all hES cell lines employed are federally approved). “The ESCRO Committee should 1. Provide oversight over all issues related to deviation and use of hES cell lines. 2. Review and approve the scientific merit of research protocols. 3. Review compliance of all in-house hES cell research with all relevant regulations and these guidelines. 4. Maintain registries of hES cell research conducted at the institution and hES cell lines derived (not relevant in Nebraska) or imported by institutional investigators. 5. Facilitate education of investigators involved in hES cell research.” The UNMC Scientific Research Oversight Committee (SROC) undertakes all of these tasks and reports its actions to the UNMC IRB and the Chancellor. # Forms * [Scientific Research Oversight Committee (SROC) Submission Form for Stem Cell Research](https://guides.unmc.edu/attachments/197) * [SROC Application for Continuing Review](https://guides.unmc.edu/attachments/198) * [SROC Request for Change in Protocol](https://guides.unmc.edu/attachments/199) # IRB Staff This section will detail contact information for both IRB Staff and IRB Chairs. # IRB Analysts/Staff |Deron Anderson, MS| |:------------------| |IRB Analyst| |402-559-5845| |[Email](mailto:deron.anderson@unmc.edu)| |Megan Berger, BS, MEd| |:---------------| |IRB Analyst & Education Coordinator |402-559-6044| |[Email](mailto:mberger@unmc.edu)| |Erika Boohar, BA| |:---------------| |IRB Analyst| |402-559-3853| |[Email](mailto:erboohar@unmc.edu)| |Bobbi Chapman, MS| |:---------------| |IRB Analyst| |402-559-6119| |[Email](mailto:bobbi.chapman@unmc.edu)| |Kristi DeHaai, MS, CIP| |:---------------| |IRB Analyst| |402-559-9991| |[Email](mailto:krdehaai@unmc.edu)| |Emily Gale, BS| |:---------------| |IRB Analyst| |402-559-7269| |[Email](mailto:emily.gale@unmc.edu)| |Bruce Gordon, MD [Biography](https://www.unmc.edu/vcr/about/leadership/assistant-regulatory-affairs.html)| |:---------------| |Assistant Vice Chancellor for Regulatory Affairs| |Executive Chair, Institutional Review Boards| |Professor, Division of Pediatric Hematology/Oncology| |402-559-6463| |[Email](mailto:bgordon@unmc.edu)| |Gail D. Kotulak, BS, CIP| |:---------------| |IRB Analyst| |402-559-6540| |[Email](mailto:gkotulak@unmc.edu)| |Sue M. Logsdon, MS, CIP| |:---------------| |IRB Analyst| |402-559-3779| |[Email](mailto:slogsdon@unmc.edu)| |Bryan Ludwig, BA, CIP| |:---------------| |IRB Analyst| |402-559-8561| |[Email](mailto:bmludwig@unmc.edu)| |Jessica Odvody-Wernimont, BS| |:---------------| |IRB Analyst| |402-559-4741| |[Email](mailto:jessica.odvody@unmc.edu)| |Nancy Olson, JD| |:---------------| |IRB Analyst| |601-946-9020| |[Email](mailto:nanolson@unmc.edu)| |Karli Storm-Narvainen, PhD| |:---------------| |IRB Analyst| |402-559-6898| |[Email](mailto:kastorm@unmc.edu)| |Nicole Weber, BA| |:---------------| |IRB Analyst| |402-559-6596| |[Email](mailto:niweber@unmc.edu)| # IRB Chairs The Institutional Review Board (IRB) is composed of members from a variety of scientific disciplines and individuals from the community who assist investigators in the protection of the rights and welfare of human subjects. ## Executive Chair *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/79nWVmI68KLA2x07-gordon-b.jpg) **Bruce Gordon, MD** Executive Chair, UNMC Institutional Review Board Chair, Joint Pediatric Institutional Review Board 402-559-6463 [Email](mailto:bgordon@unmc.edu) [Biography](https://www.unmc.edu/vcr/about/leadership/assistant-regulatory-affairs.html) ## Chairs [](https://guides.unmc.edu/uploads/images/gallery/2025-02/2rhnshHEvKRibQt1-beam200.png) **Elizabeth Beam, PhD, RN** Vice Chair, IRB-01 | Vice Chair, IRB-05 UNMC Institutional Review Board 402-559-6547 [Email](mailto:ebeam@unmc.edu) *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/oGtauysmy91oH1BL-lunning-125.jpg) **Matthew Lunning, DO** Vice Chair, IRB-01 | Vice Chair, IRB-05 UNMC Institutional Review Board 402-559-7164 [Email](mailto:mlunning@unmc.edu) *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/E3yKmeenKceI1yXR-amacfadyen-125.jpg) **Andrew Macfadyen, MD** Vice Chair, Children's Nebraska Joint Institutional Review Board UNMC Institutional Review Board 402-955-4235 [Email](mailto:amacfadyen@unmc.edu) *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/z7xkwFxHh7obr8YQ-sue-nuss-125.jpg) **Suzanne Nuss, RN, MBA, PhD** Vice Chair, IRB-03 | Chair, IRB-05 UNMC Institutional Review Board 402-559-8815 [Email](mailto:slnuss@nebraskamed.com) *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/cqzxYtZqhN07LbEs-snyder-125.jpg) **Sheilah Snyder, MD** Chair, Children's Nebraska Joint Institutional Review Board UNMC Institutional Review Board 402-955-8125 [Email](mailto:sjsnyder@unmc.edu) *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/MPtatSkW08MXX0e5-stoller-125.jpg) **Douglas A. Stoller, MD, PhD** Vice Chair, IRB-01 UNMC Institutional Review Board 402-559-1350 [Email](mailto:douglas.stoller@unmc.edu) *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/3kEft8YNiXAA5KAk-ben-teply-125.png) **Benjamin Teply, MD** Vice Chair, IRB-02 UNMC Institutional Review Board 402-559-7915 [Email](mailto:ben.teply@unmc.edu) *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/5Ua47PCmkvqE6UN6-vaughn-b.jpg) **Brigette Vaughan, APRN** Chair, IRB-02 UNMC/Children's Nebraska Joint Institutional Review Board 402-559-9800 [Email](mailto:bvaughan@unmc.edu) *** [](https://guides.unmc.edu/uploads/images/gallery/2025-02/mNcWZ7M3QM4vcM2r-zeger-w.jpg) **Wes Zeger, DO** Vice Chair, IRB-03 UNMC Institutional Review Board 402-559-6841 [Email](mailto:wzeger@unmc.edu)