Frequently Asked Questions (FAQ)
Below are a number of commonly asked questions. The questions will either provide an answer or will link to the appropriate section of the guidebook. The Page Navigation found in the upper left side of the page can be used to scroll directly to the answer needed and are organized by question. If you do not see your questions listed below, the Book Navigation on the lower left side of the page can be used to browse the various sections. The search bar at the top of the page may also help you navigate to the appropriate section.
Feel free to contact us at irbora@unmc.edu if you cannot find the needed content.
Do I need IRB review?
What application should I use?
What does Exempt mean?
The term Exempt candoes sometimesnot bemean misunderstoodexempt infrom regardsan toethics review, but rather exempt from the IRB.federal Whenregulations. Research is exempt from the IRBfederal usesregulations theonly wordif Exempt in reference to a research project, that means a projectit falls under a certain setone of criteria for Human Subject Research. Often times, the IRBspecific willexemption becategories askedlaid ifout ain project45 isCFR exempt meaning: is my project considered Human Subject Research that the IRB needs to review my project?46.
- Information regarding Exempt category research can be found here: HRPP 2.6 (Exempt Research)
- If you would like to know if your project requires IRB review, please use our Determination Survey.
Is my research project expedited or full board?
The determination of whether a project will be considered Expedited or Full Board will be made by the IRB analyst during the review of the application. If you have a application deadline that requires an expedient review, this can be notated in the IRB application itself in Section 1.4:
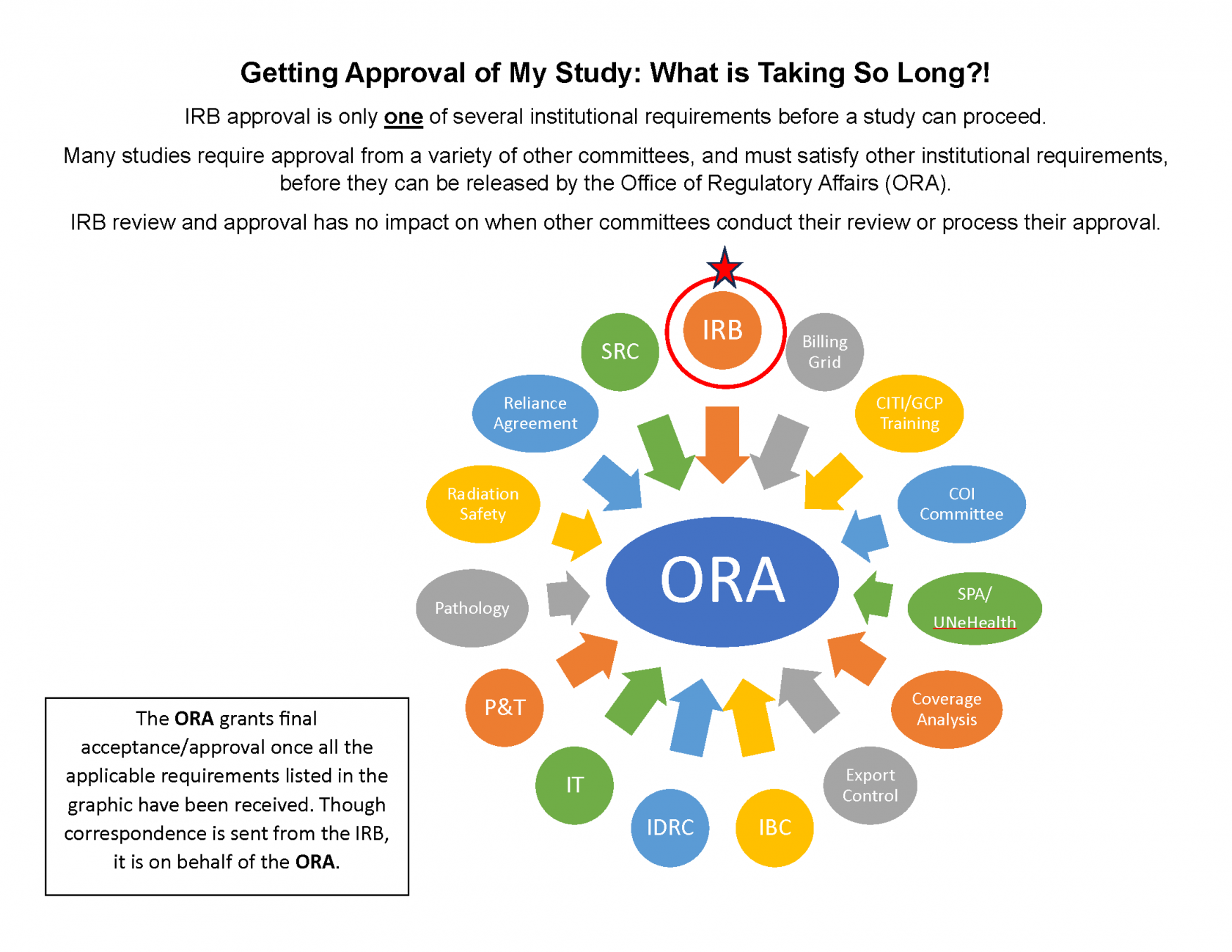
How long does it take for my research project to get approved?
There are many factors involved in IRB review depending on the complexity of the application and how many departments in the Office of Regulatory Affairs must be involved. On average an application will be reviewed between 7 and 28 working days.
I have a CITI question.
I have an RSS question.
My protocol closes soon, what do I do?
Any protocol which needs to be renewed for another year will fall under one of two scenarios: Continuing Review (Full Board or greater than minimal risk studies) or a Demographic Recruiting Numbers form (EX, EP, CB, and minimal risk). Each will have their own respective form to be completed.
- The Continuing Review period will begin two months prior to the expiration of a study. Notifications will be sent out 60 and 45 days prior to the expiration of the protocol.
- The Demographic Recruiting Numbers form will be generated on the first day of the month a study expires.
- RSS will generate an email notification sent to the PI and Lead Coordinator for both types of renewal.
How do I complete a Continuing Review?
Instructions will be coming soon.
Do I need data from other sites on my Continuing Review (CR)?
- For local studies and cIRB studies - only report UNMC data in the CR.
- For sIRB studies - please contact sirb@unmc.edu
How do I know my Accrual Numbers?
New study applications - when providing information for how many participants are going to be recruited, please provide a solid number value. Avoid using phrases like "approximately" or "around". It is acceptable to provide a larger number than anticipated as investigators can always accrue less participants than applied for, but going above the stated accrual numbers requires submission of a Request for Change and IRB review of stated change. Please take into account that screen failures and withdrawls count as accrual.
Continuing Review/Study Closure/Demographic Recruiting Numbers form - during the yearly renewal (or closure) phase of an investigator's protocol, demographics are often required as part of the process. If demographics are recorded as part of your project, it is advisable to document this information as participants are recruited making this information readily available when reporting is necessary.
How do I close my study?
- For studies that require Continuing Review that needs to be closed, a Study Completion Report can be created using the Forms button in RSS.
- For studies that require Demographic Recruiting Numbers forms to be closed:
- If the study is in the month of expiration and the form has already been generated, the form can be used to mark the study as Complete.
- If the study is NOT in the month of expiration, send a message to the IRB via the RSS message portal for that protocol. The message will act as official documentation of the request and the IRB will complete the process.
- For cIRB studies: The formal closure of a study is done through the IRB of record. All that needs to be done locally is to send a message through the message portal to the analyst requesting that the application be set to closed. Feel free to upload any pertinent documentation, though not required.
What do I do if I have a problem?
- An investigator can always reach out to the IRB or their study analyst by using the Message Portal in RSS for any particular protocol.
- If the answer to your question cannot be found in the IRB Guidebook, please email irbora@unmc.edu
How often does the IRB meet?
The IRB Boards meet once per month.
- IRB-01 (Adult; first Thursday of every month except January and July)
- IRB-02 (Adult; third Thursday of every month)
- IRB-04 (Pediatric; fourth Tuesday of every month)
- IRB-05 (SIRB; second Friday of every month)
How do I delete a document or consent form?
Once a document or consent form has been deleted, it will be irretrievable. To avoid any accidental deletion of these documents, the request must be performed by a member of the IRB team. Please contact us through the RSS Message portal or at irbora@unmc.edu if this needs to be done.
How do I make a Change Request?
How do I reset my application to edit?
When going into the protocol in RSS, the Reset Edit button should be available at the top of the protocol page. If this does not appear, contact irbora@unmc.edu and the IRB can change the status.
I'm a student, who needs to be listed on my protocol?
The student must be listed as the PI of the project, while the student's faculty advisor must be listed as both Secondary Investigator and Faculty Advisor. The Faculty Advisor role in RSS will appear when question 2.G in the application is answered "Yes".
- If multiple students are involved in a project, only one may be listed as PI. All other students will fill the role of Secondary Investigator. The PI will be the only role capable of signing off on the application and any changes that may occur.
How do I edit a consent form?
I'm leaving UNMC, what do I do with my research before I leave?
- The first option would be to change the Principal Investigator. Often, a Secondary Investigator can be approached to take responsibility for the protocol.
- If no replacement Prinicpal Investigator can be found, the protocol should be closed. Help closing a protocol can be found here.
I have a cIRB/sIRB question.
My project was closed, what do I do?
Please contact irbora@unmc.edu with the protocol number. The circumstances of when and why a protocol was closed will depend on if and how the protocol can be reopened.
How does ClinicalTrials.gov work?
How do I compensate my participants?
I'm trying to add someone to my study in RSS, but I don't see their name.
All UNMC personnel will automatically receive an RSS profile with the creation of their institutional email address. If new employees do not appear in RSS within 48 hours of the creation of their email, please contact irbora@unmc.edu
For local institutions (UNO, UNL, and Creighton), when going to https://net.unmc.edu/rss/ choose the appropriate institution portal. This will redirect you to login with your institutional credentials and will sync your access with UNMC's RSS system.
For external institutions, please contact irbora@unmc.edu to determine the best course.
What do I need to report with an Incident Report?
How do I know if I need a Data Use Agreement?
When investigators are planning to share or receive data, two things must be considered:
- Is this Research – as determined by the IRB? and
- Does the data include Protected Health Information as defined under HIPAA?
If both of those answers are “yes” it will be necessary to proceed to a DUA.
If it is not research, but rather Quality Improvement (as determined by the IRB) and PHI is being shared, then the Privacy Office will be engaged to determine if a DUA is needed.
If de-identified data is being shared, the IRB does not require a DUA, however one still may be issued if 1) the investigator would like one in place to govern how the data is managed and/or 2) the other institution requires one to be in place.


